Heat is a form of energy which makes the substance hot. In winter, it is our common experience that we feel cold inside the house and if we come out in front of sun rays, then we feel warm. Now, if we know that how do we feel this sensation of warm or cold? Then, what will be our answer? Think. In this chapter, we will try to find out the answer to such kind of question.
Hot and Cold
In our daily routine, we come across a number of objects, out of which some are hot while other objects are cold, e.g. when a frying pan kept on a burning gas stove becomes hot but the handle of the pan is cold. Even among the hot objects, some objects may be hotter than the other. In the same manner, among the cold objects, some objects may be colder than the other. So, if I ask you how you decide the relative hotness or coldness of objects, then your answer will be’by simply touching the objects’. But our sense of touch is not enough in telling us whether an object is really hot or cold so, this can be understood by performing a simple activity.
Temperature and Thermometer
The degree of hotness or coldness of the object is known as the temperature of an object. The temperature of an object is an only property that indicates which object is hot and which one is cold. A high temperature of a body indicates that it is very hot whereas a low temperature of the object indicates that it is quite cold, e.g. the temperature of boiling water is quite high, so boiling water appears to be very hot. On the other side, the temperature of melting ice is quite low. So, ice appears to be very cold on touch.
It is measured by using an instrument called thermometer, which has a scale marked on it which is used to read the temperature, e.g. the scale in laboratory thermometer is marked along the length of thermometer’s tube between 0° mark and 100° mark into 100 equal divisions. So, each division is called a degree. The temperature of an object should always be stated with its unit. So, the most common unit for measuring temperature is degree Celsius (°C).
Both the clinical thermometer and laboratory thermometer are mercury thermometers. So, when a particular amount of heat is supplied to the thermometer bulb consisting of mercury (by the hot body whose temperature is to be measured), then the mercury expands and get rises in the glass tube of the thermometer. This fact is used in measuring the temperature.
Clinical Thermometer
It is the thermometer which is used for measuring the temperature of the human body. In case of fever, it is used by a doctor (or at home) to measure the temperature of the patient. This thermometer consists of a long glass tube having a thin and uniform bore. There is a glass bulb at one end of the glass tube which consists of mercury as shown in the figure given below:
Features of a Clinical Thermometer
There is a very short range of temperature of a clinical thermometer, i.e. from 35°C to 42°C. The short range of a clinical thermometer is because of the fact that the temperature of human body normally does not go below 35°C or above 42°C.

Just above the bulb containing mercury, a clinical thermometer has a kink in its glass tube which is to prevent the back flow of mercury into the thermometer bulb when the thermometer bulb is removed from the mouth of a patient. This kink prevents the mercury level in the thermometer tube from falling on its own. Due to this, we can read the correct body temperature of the patient even after removing the thermometer bulb from his mouth.
Note: After noting the body temperature, the level of mercury can be brought down by giving jerk to the thermometer tube.
As mercury is very toxic and is difficult to dispose off, so thermometer must be handled carefully. Clinical thermometer should not be used to measure the temperature of objects other than the human body. It should not be kept in the sun or near a flame, otherwise, it may break. Nowadays, digital thermometers are used which do not use mercury.
Reading a Clinical Thermometer
There are following steps to read the temperature on a thermometer.
Step I: Firstly, wash the thermometer with an antiseptic solution and if in case, the antiseptic solution is not available, then wash it with clean water.
Step II: Gently, hold the thermometer tube in your hand and give it a jerk in such a way that the mercury thread in the thermometer tube falls below the reading of 35°C.
Step III: Now, put the bulb of the thermometer under the tongue of the patient for about one minute. Then take out the thermometer from the patient’s mouth.
Step IV: In order to read the temperature, hold the thermometer horizontally in your hand and rotate it slowly. When we see a magnified image of the mercury thread in its tube, then a position will come. Now, read the temperature on thermometer tube in level with the top of the mercury thread.
Precautions while Reading the Thermometer
A clinical thermometer should not be used for any object other than the human body. There are some following precautions which are to be observed while reading a clinical thermometer.
- Wash the clinical thermometer before and after using preferably with an antiseptic solution.
- Be ensure that the mercury level before using the clinical thermometer should be below 35°C.
- The clinical thermometer should be read by keeping the level of mercury along the line of sight.
- While reading the clinical thermometer, it should never be held by the bulb.
- The clinical thermometer should be carefully handled.
Laboratory Thermometer
A device which is used for measuring the temperature in a science laboratory is called a laboratory thermometer.
This thermometer is made up of a long glass tube having a thin bore. The graduation marked on the tube of a laboratory thermometer can measure the temperature from -10°C to 110°C, this is known as the range of a laboratory thermometer. Also, determine how much a small division on this thermometer reads (this is also known as least count of the thermometer), it is due to the fact that this information is required to read the thermometer correctly.

These are the special thermometers which automatically record the maximum and minimum temperature of the day. The maximum S and minimum temperature of the last day reported in weather reports in TV and newspapers are measured by the maximum-minimum thermometers.
Reading a Laboratory Thermometer
There are following steps to read the temperature on a thermometer.
Step I: First of all, take some hot water in a beaker.
Step II: Now, try to hold the laboratory thermometer from its glass tube and immerse the bulb of the thermometer in hot water taken in the beaker. Notice that the bulb of the thermometer should not touch the sides or the bottom of the beaker as shown in the figure.
Step III: Here, we will observe the shining thread of mercury moving up in the thermometer tube. After some time, the mercury will stop rising and stand at one place.
Now, read the temperature on the thermometer tube which corresponds to the top of the mercury thread. This will give us the temperature of hot water taken in the beaker.
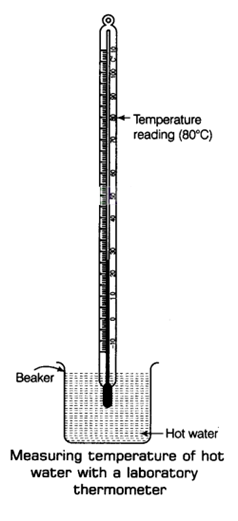
Note: To measure the human body temperature a laboratory thermometer cannot be used because as soon as we tahe out the bulb of the laboratory thermometer from the mouth of a patient, the mercury level wilt starts falling quickly (due to cooling of its bulb by air). So, this will provide a wrong value of the body temperature.
Precautions in Using a Laboratory Thermometer
- While -measuring temperature, the laboratory thermometer should be held vertically.
- The thermometer bulb should be surrounded from all sides by the substance whose temperature is to be measured.
- The thermometer reading should be taken while its bulb is still in touch with the substance whose temperature is being measured and by keeping the mercury level along the line of sight.
- The thermometer should not be held by the bulb.
- The thermometer should be carefully handled.
- We should note down the temperature reading by keeping the thermometer bulb immersed in hot water because if the thermometer bulb is taken out of hot water, then its mercury thread will start falling and this will give a wrong reading for the temperature of hot water.
Digital Thermometer
There are most of the common thermometers like mercury thermometers which use a liquid metal called mercury for their working. Mercury is a toxic substance (a poisonous substance) and thus it is very difficult to dispose of safely, if a thermometer breaks. So, there is a lot of concern over the use of mercury in thermometers. Also, during these days, digital thermometers are available which do not use mercury.
Transfer of Heat
Heat flows from a hot object to a cold object or heat flows from an object at the higher temperature to another object which is at a lower temperature. This flow of heat is known as the transfer of heat, e.g. if you dip a steel spoon into a cup of hot tea, then we will find that the temperature of spoon rises and it becomes hot. In this case, some of the heat contained in hot tea has been transferred to spoon which is placed inside it.
When the two objects attain the same temperature, then the flow of heat stops. This means that no heat will be transferred from one object to another if the temperature of the two objects is the same.
There are three ways through which heat can be transferred from a hot object to a cold object.
- By conduction (in solid, heat is transferred by conduction)
- By convention (in liquid and gases, heat is transferred by convection)
- By radiation (in free space or vacuum, heat is transferred by radiation)
Let us discuss all the three ways of heat transfer.
1. Conduction
The mode of transfer of heat from hotter part of a material to its colder part or from a hot material to a cold material in contact with it, without the movement of material as a whole, is known as conduction. In all the solids, heat is transferred by the process of conduction.
Conductor and Insulator of Heat
Materials which allow heat to be conducted through them easily are conductors of heat. Those metals such as iron, copper, silver, aluminium, etc., are good conductors of heat.
Bad conductors of heat are those materials which do not allow heat to be conducted through them easily. These materials are also known as insulators of heat. Wood, plastic and glass are insulators of heat.
Uses of Good and Bad Conductors of Heat
During the winter season, we generally wear woollen clothes. If we compare them with cotton clothes, then we will find that the wool fibres have much more space between them. These get filled with air which is a bad conductor of heat. Hence, being an insulator, both wool and air together prevent the heat from our bodies from escaping out.
Also, jute and sawdust are bad conductors of heat. We cover the ice with a jute cloth of sawdust to prevent it from gaining heat from the surroundings and melting.
The double walls of the refrigerators having space inside which is filled with an insulating material, prevent the heat of the surroundings from reaching the inside of the refrigerator.
The two thinner blankets (one on top of the other) during the winter season are very much effective because the air layer trapped between the thinner blankets creates insulation and provides the protection from cold.
Sometimes, there are two things which are at the same temperature. It seems like they are at different temperatures, one being cold and the other being warm. This happens because some things are a good conductor of heat while others are poor conductors of heat.
e.g. during winter season, a metal object kept in a room feels very cold to touch but a wooden object in the same room feels warmer to touch. Metal object is a good conductor of heat. So, when we touch the metal object, it conducts away heat from our hand quickly. And by losing heat, our hand feels cold. On the other side, the wooden object (being a poor conductor of heat) does not allow the heat of our hand to escape and hence feels warmer to touch.
The water (or most liquids) and air (or gases) are bad conductors of heat.
2. Convection
The mode of transfer of heat from the hotter part of a fluid (liquid or gas) to its colder parts by the movement of the liquid (or gas) itself is known as convection. The transfer of heat by convection can take place only in liquids and gases. It is due to the reason that the particles in liquids and gases can move about freely.
So, the transfer of heat by convection cannot take place in solids because the particles in the solids are fixed at a place and cannot move about freely. It is also not occurred in empty space or vacuum because there are no particles of any kind in empty space which can move and transfer heat.
Convection in Water
Water is a poor conductor of heat. So, due to this reason, it cannot transfer heat by conduction but it transfers heat by the process of convection.
Convection in Air
Air is a very poor conductor of heat, Air transfers heat from its hotter parts to the colder parts by the process of convection.
Sea and Land Breezes
The blowing of sea breeze and land breeze in coastal areas is generally occurred due to the convection of heat in air.
In coastal areas during the day time, the breeze generally flows from the sea towards the land and during the night time, blows from the land towards the sea. Sea and land breezes are actually convection of heat.
During the day, the land heats up more than water. Due to this, the air over the land becomes hotter and lighter and rises up. So, the air from the sea which is cooler and heavier rushes to take the place created by hot rising air. Therefore, a sea breeze blows during the day.
During the night, the land loses heat faster than water and becomes cooler and the air over the sea is now warmer due to which, it rises up and the cooler air over the land rushes to take its place. Therefore, we observe a land breeze at night.
3. Radiation
The mode of transfer of heat through which heat energy from a hot body to a cold body by means of heat rays without any material medium between them is known as radiation, e.g. the sun’s heat reaches the earth by the process of radiation. The sun is very far away from the earth, and there is mainly an empty space (vacuum) between the sun and the earth even, then the heat from the sun reaches the earth. This is due to the fact that the sun being extremely hot, emits invisible heat radiation (or infrared rays) in all directions.
These radiations travel through the vacuum between the sun and the earth at a very high speed and ultimately, reach us on the earth. Therefore, we can say that the transfer of heat from a hot object to a cold object by the process of radiation does not require any medium.
In our daily life activities, we have many situations where heat is transferred by radiation through air, e.g.
- Depending on the temperature of surroundings, our body too gives heat to the surroundings or receives heat from the surroundings by radiation.
- If a hot utensil filled with hot milk is kept away from the flame, then it cools down by transferring its heat to the surroundings by radiation.
- If we stand next to a burning fire, then we will feel the heat of the fire falling on our face. The heat is transferred from the fire to our face by the process of radiation.
Clothes
During hot summer days, people prefer to wear white clothes or light coloured clothes because light coloured clothes absorb less heat from the sun and hence, keep us cool and comfortable in hot weather while in the cold winter days people prefer to wear dark clothes because the dark coloured clothes absorb more heat rays from the sun and keep us warm in winter season.
Thus, we can say that dark coloured objects absorb heat better and also emit heat better than light coloured objects. Now, let us try to study this concept on the basis of the given activity.
In the winters, we use woollen clothes. Wool is a poor conductor of heat. Moreover, there is air trapped in between the wool fibres. This air prevents the flow of heat from our body to the cold surroundings. So, we feel warm.