We, at cbseinsights.com have prepared the exhaustive content for the Class 10 Chemistry Periodic Classification of Elements in form of the Questions and Answers in order to help students with the latest material for CBSE 2021-22. As per the present situation of Pandemic of Covid, it is best platform to study and practice hard for the Competitive Exams.
Multiple Choice Questions 1 Marks
Q 1 – On moving from left to right in a period :
(a) electropositive nature of elements increases
(b) metallic character of elements increases
(c) The ionic radius of elements increases
(d) The basic nature of oxides of elements decreases.
Q 2 – One of the defects of Mendeleev’s periodic table is that:
(a) halogens are placed in the same group
(b) on its basis properties of known elements cannot be predicted
(c) elements of dissimilar properties are placed in the same group
(d) periodic table is not based on any fundamental principle.
Q 3 – The number of groups in Mendeleev’s periodic table is:
(a) 7
(b) 8
(c) 9
(d) 16
Q 4 – The properties of eka-aluminium predicted by Mendeleev are the same as the properties of later discovered element:
(a) Scandium
(b) Germanium
(c) Gallium
(d) Aluminium
Q 5 – Modern periodic law is given by:
(a) Mendeleev
(b) Moseley
(c) Priestly
(d) Davy
Q 6 – An atom of an element has the electronic configuration 2, 8, 2. To which group does it belong?
(a) 4th group
(b) 6th group
(c) 3th group
(d) 2th group
Q 7 – Element with 18 atomic number is in:
(a) zero group
(b) VIII group
(c) period fourth
(d) transition series
Cbseinsights.com
Q 8 – Which of these belong to the same period?
| Element | A | B | C |
| Atomic number | 2 | 10 | 5 |
(a) A, B
(b) B, C
(c) C, A
(d) A, B and C
Q 9 – (i) Li and (ii) Be show diagonal relation with:
(a) (i) Mg and (ii) Al
(b) (i) Na (ii) Mg
(c) (ii) K and (ii) B
(d) (i) Na (ii) K
Q 10 – Which one of the following statements is not correct about the trends in the properties of the elements of a period on going from left to right?
(a) The oxides become more acidic
(b) The elements become less metallic
(c) There is an increase in the number of valence electrons
(d) The atoms lose their electrons more easily
Q 11 – Element with atomic number 17 is placed in:
(a) VII period, VII group
(b) III period, VII group
(c) IV period, VII group
(d) II period, VI group
‘Q 12 –Why do group I elements form unipositive ions?
Q 13 – Formula of chloride of an element is MCl2. Write the formula of its oxide.
(a) MO2
(b) MO
(c) M2O3
(d) M2O
Q 14 – Consider the following elements
Ca, O, Ar, S, Be, He
Which of the above elements would you expect to be in group 16 of the Periodic Table?
(a) Ca and S
(b) Ca and O
(c) Ar and S
(d) O and S
Short Answer Type Questions 2 Marks
Q 14 – Which group of elements could be placed in Mendeleev’s Periodic Table without disturbing the original order? Give reason.
Q 15 – How does the metallic character change along the period ?
Q 16 – Elements have been arranged in the following sequence on the basis of their increasing atomic masses.
(a) Pick two sets of elements which have similar properties.
(b) The given sequence represents which law of classification of elements?
Q 17 –What is the Newlands’ Law of Octaves.
Q 18 –What do you understand by the term periodicity? Does the periodicity in properties is a function of valence electrons? Illustrate.
Q 19 –Define atomic radius? Why does atomic radius decrease across a period?
Q 20 – Can the following groups of elements be classified as Dobereiner’s triad?
(a) Na, Si, ci
(b) Be, Mg, Ca
Atomic mass of Be- 9 ; Na- 23 ; Mg- 24 ; Si-28 ; Cl-35 ; Ca- 40 . Explain by giving reason.
Q 21 – An element P (atomic number 20) reacts with an element Q (atomic number 17) to form a compound. Answer the following questions giving reason: Write the position of P and Q in the Modern Periodic Table and the molecular formula of the compound formed when P reacts with Q
Q 22 – The elements Be, Mg and Ca, each having two valence electrons in their valence shells are in periods 2, 3 and 4 respectively of the Modern Periodic Table. Answer the following questions associated with these elements, giving reason in each case :
(a) In which group should they be?
(b) Which one of them is least reactive?
(c) Which one of them has the largest atomic size?
Long Answer Type Questions 3-4 Marks
Q 23 – Two elements ‘P’ and ‘Q’ belong to the same period of the Modern Periodic Table and are in Group-1 and Group-2 respectively. Compare their following characteristics in tabular form:
(a) The number of electrons in their atoms
(b) The sizes of their atoms
(c) Their metallic characters
(d) Their tendencies to lose electrons
(e) The formula of their oxides (f) The formula of their chlorides
Q 24 – The atomic number of nitrogen, oxygen and fluorine are 7, 8 and 9 respectively. Write the electronic configuration of each element and answer the following questions:
(a) Which one of N, O and F is most electronegative and which on is least electronegative?
(b) What is the number of valence elecrtons of F?
(c) What is valency of each one of N, O and F?
Q 25 – (a) How would the tendency to lose electrons change as you go :
(i) from left to right across a period?
(ii) down a group?
(b) An element X (2, 8, 2) combines separately with , and radicals. Write the formulae of the three compounds so formed. To which group of the periodic table does the element ‘X’ belong? Will it form covalent or ionic compound? Why?
Q 26 – (a) How does the atomic radius change as you go :
(i) from left to right in a period?
(ii) down a group in the periodic table?
(b) Two elements X and Y have atomic numbers 12 and 16 respectively.
Write the electronic configuration for the these elements. To which period
of the Modern Periodic Table do these two elements belong? What type of bond will be formed between them and why?
Comprehensive Type Questions
Q 27 – Read the following and answer any four questions from 18 (1) to 18 (5). Metallic Character The ability of an atom to donate electrons and form positive ion (cation) is known as electropositivity or metallic character. Down the group, metallic character increases due to increase in atomic size and across the period, from left to right electropositivity decreases due to decrease in atomic size. Non-Metallic Character The ability of an atom to accept electrons to form a negative ion (anion) is called non-metallic character or electronegativity. The elements having high electro-negativity have a higher tendency to gain electrons and form anion. Down the group, electronegativity decreases due to increase in atomic size and across the period, from left to right electronegativity increases due to decrease in atomic size.
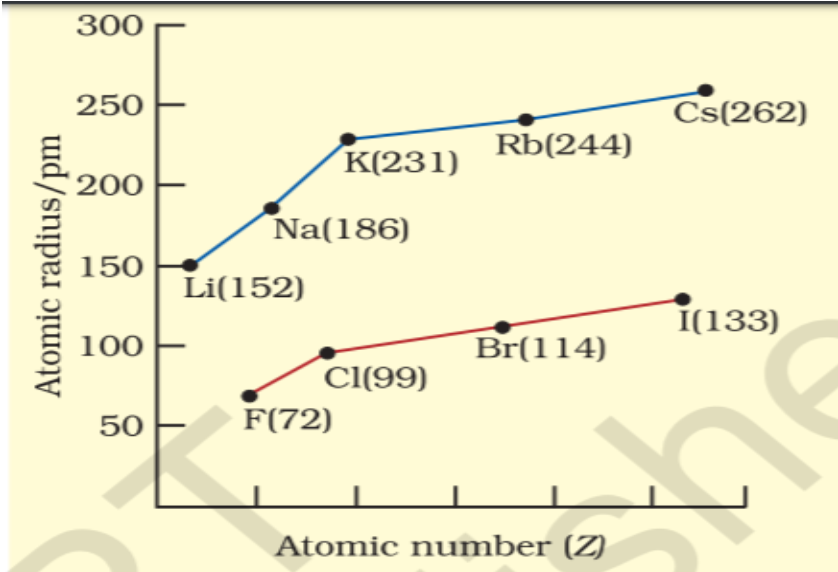
Cbseinsights.com
- Which of the following correctly represents the decreasing order of metallic character of Alkali metals plotted in the graph?
a) Cs > Rb > Li > Na > K
b) K > Rb > Li > Na > Cs
c) Cs > Rb > K > Na > Li
d) Cs > K > Rb > Na > Li - Hydrogen is placed along with Alkali metals in the modern periodic table though it shows non-metallic character
a) as Hydrogen has one electron & readily loses electron to form negative ion
b) as Hydrogen can easily lose one electron like alkali metals to form positive ion
c) as Hydrogen can gain one electron easily like Halogens to form negative ion
d) as Hydrogen shows the properties of non-metals - Which of the following has highest electronegativity?
a) F
b) Cl
c) Br
d) I - Identify the reason for the gradual change in electronegativity in halogens down the group.
a) Electronegativity increases down the group due to decrease in atomic size
b) Electronegativity decreases down the group due to decrease in tendency to lose electrons
c) Electronegativity decreases down the group due to increase in atomic radius/ tendency to gain electron decreases
d) Electronegativity increases down the group due to increase in forces of attractions between nucleus & valence electrons - Which of the following reason correctly justifies that “Fluorine (72pm) has smaller atomic radius than Lithium (152pm)”? a) F and Li are in the same group. Atomic size increases down the group
- b) F and Li are in the same period. Atomic size increases across the period due to increase in number of shells
- c) F and Li are in the same group. Atomic size decreases down the group
- d) F and Li are in the same period and across the period atomic size/radius decreases from left to right.