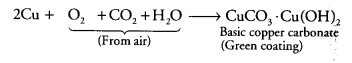Question 1
Give an example of a metal which :
(i) is a liquid at room temperature.
(ii) can be easily cut with a knife.
(iii) is the best conductor of heat.
(iv) is a poor conductor of heat.
(i) Mercury
(ii) Sodium
(iii) Silver
(iv) Lead
Question 2
Explain the meanings of malleable and ductile.
Malleable : A metal that can be beaten into thin sheets on hammering is called malleable.
Ductile : A metal which can be drawn into thin wires is called ductile.
Question 3
Why is sodium kept immersed in kerosene oil ?
Sodium is highly reactive. So it is kept immersed in kerosene oil to prevent its reaction with oxygen, moisture and carbon dioxide of air to prevent accidental fires.
Question 4.
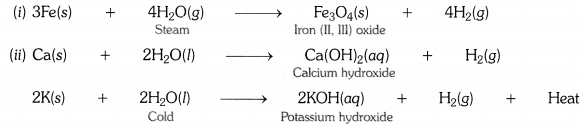
Write equations for the reactions of
(i) iron with steam.
(ii) calcium and potassium with water.
Question 5
Samples of four metals A, B, C and D were taken and added to the following solution one by one.
The results obtained have been tabulated as follows :
| Metal | Iron (II) sulphate | Copper (II) sulphate | Zinc sulphate | Silver nitrate |
| A | No reaction | Displacement | ||
| B | Displacement | No reaction | ||
| C | No reaction | No reaction | No reaction | Displacement |
| D | No reaction | No reaction | No reaction | No reaction |
Use the Table above to answer the following questions about metals A, B, C and D.
(i) Which is the most reactive metal ?
(ii) What would you observe if B is added to a solution of copper (II) sulphate?
(iii) Arrange the metals A, B, C and D in the order of decreasing reactivity.
(i) B is the most reactive metal because it gives displacement reaction with iron (II) sulphate.
(ii) When metal B is added to copper (II) sulphate solution, a displacement reaction will take place due to which the blue colour of copper (II) sulphate solution will fade and a red-brown deposit of copper will be formed on metal B.
(iii) Metal B is the most reactive because it displaces iron from its salt solution. Metal A is less reactive because it displaces copper from its salt solution. Metal C is still less reactive because it can displace only silver from its salt solution and metal D is the least reactive because it cannot displace any metal from its salt solution. Hence, the decreasing order of reactivity of the metals is B > A > C > D.
Question 6
Which gas is produced when dilute hydrochloric acid is added to a reactive metal ? Write the chemical reaction when iron reacts with dilute H2SO4.
Hydrogen gas is produced when dilute hydrochloric acid is added to a reactive metal.
Chemical reaction when iron reacts with dilute H2SO4 :
Fe(s) + H2SO4(aq) → FeSO4(aq) + H2(g)
Question 7.
What would you observe when zinc is added to a solution of iron (II) sulphate ? Write the chemical reaction that takes place.
Zinc is more reactive than iron. Therefore, when zinc is added to a solution of iron (II) sulphate, then the greenish colour of iron (II) sulphate solution fades gradually due to the formation of colourless zinc sulphate solution and iron metal is deposited on zinc.
Question 8
(i) Write the electron dot structures for sodium, oxygen and magnesium.
(ii) Show the formation of Na2O and MgO by the transfer of electrons.
(iii) What are ions present in these compounds?

(ii) Formation of Na2O and MgO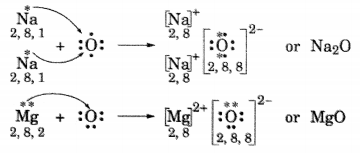
(iii) In Na2O, ions present are Na+ and O2-.
In MgO, ions present are Mg2+ and O2-.
Question 9
Why do ionic compounds have high melting points ?
(iii) What are ions present in these compounds?
The ionic compounds are made up of positive and negative ions. There is a strong force of attraction between the oppositely charged ions, so a lot of heat energy is required to break this force of attraction and melt the ionic compound. Due to this, ionic compounds have high melting points.
Question 10.
Define the following terms :
(i) Mineral : The natural materials in which the metals or their compounds are found in earth are called minerals.
(ii) Ore : Those minerals from which the metals can be extracted conveniently and profitably are called ores.
(iii) Gangue : The unwanted impurities like sand, rocky material, earth particles, lime stone, mica, etc in an ore are called gangue.
Question 11.
Name two metals which are found in nature in the free state.
Gold and platinum
Question 12
What chemical process is used for obtaining a metal from its oxide.
Reduction process is used for obtaining a metal from its oxide.
For example, zinc oxide is reduced to metallic zinc by heating with carbon.
ZnO(s) + C(s) → Zn(s) + CO(g)
Besides carbon, highly reactive metals like sodium, calcium, aluminium etc. are used as reducing agents. These displace metals of low reactivity from their oxides.
For example,
Fe2O3(s) + 2Al(s) → 2Fe(l) + Al2O3(s) + Heat
Question 13
Metallic oxides of zinc, magnesium and copper were heated with the following metals :
| Metal | Zinc | Magnesium | Copper | |
| 1. | Zinc oxide | |||
| 2. | Magnesium oxide | |||
| 3. | Copper oxide |
In which cases will you find displacement reactions taking place ?
A more reactive metal can displace a less reactive metal from its oxide. But out of zinc, magnesium, and copper metals, magnesium is the most reactive, zinc is less reactive whereas copper is the least reactive metal.
The displacement will take place in the following cases :
| Metal | Zinc | Magnesium | Copper | |
| 1. | Zinc oxide | – | Displacement | – |
| 2. | Magnesium oxide | – | – | – |
| 3. | Copper oxide | Displacement | Displacement |
Question 14
Which metals do not corrode easily ?
Gold and Platinum.
Question 15
What are alloys ?
An alloy is a homogeneous mixture of two or more metals, or a metal and a non-metal. For example, bronze is an alloy of copper and tin.
Question 16.
Which of the following pairs will give displacement reactions ?
(a) NaCl solution and copper metal.
(b) MgCl2 solution and aluminium metal.
(c) FeSO4 solution and silver metal.
(d) AgNO3 solution and copper metal.
(d) AgNO3 solution and copper metal.
Question 17.
Which of the following methods is suitable for preventing an iron frying pan from rusting ?
(a) Applying grease
(b) Applying paint.
(c) Applying a coating of zinc
(d) All the above.
(c) Applying a coating of zinc.
Question 18.
An element reacts with oxygen to give a compound with a high melting point. This compound is also soluble in water. The element is likely to be
(a) calcium
(b) carbon
(c) silicon
(d) iron
(a) Calcium.
Question 19.
Food cans are coated with tin and not with zinc because
(a) zinc is costlier than tin
(b) zinc has a higher melting point than tin
(c) zinc is less reactive than tin
(d) zinc is more reactive than tin.
(d) Zinc is more reactive than tin.
Question 20.
You are given a hammer, a battery, a bulb, wires and a switch.
(a) How could you use them to distinguish between samples of metals and non-metals?
(b) Assess the usefulness of these tests in distinguishing between metals and non-metals.
(a) Metals can be beaten into thin sheets with a hammer without breaking. Non-metals cannot be beaten with a hammer to form thin sheets. Non-metals break into pieces when hammered. Metals are malleable, while non-metals are non-melleable. When metals are connected into circuit using a battery, bulb, wires and switch, current passes through the circuit and the bulb glows. When non-metals (like sulphur) are connected, the bulb does not light up at all. Metals are good conductors of electricity.
(b) Because of malleability, metals can be casted into sheets. Metals are good conductors of electricity so these can be used for electrical cables.
Question 21.
What are amphoteric oxides ? Give two examples of amphoteric oxides ?
OR
Write chemical equations that show aluminium oxide reacts with acid as well as base.
Those metal oxides which show basic as well as acidic behaviour are known as amphoteric oxides. In other words, metal oxides that react wtih both acids and bases to form salt and water are called amphoteric oxides. Aluminium oxide and zinc oxide are amphoteric in nature.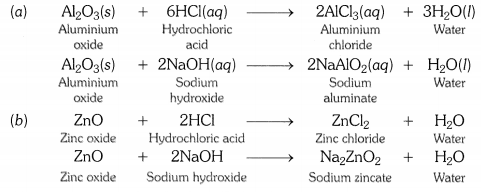

Question 22.
Name two metals which will displace hydrogen from dilute acids and two metals which will not.
(i) Metals above hydrogen in the activity series like sodium and magnesium displace hydrogen from dilute acids.
(ii) Metals below hydrogen in the activity series like copper, silver do not displace hydrogen from dilute acids.
Question 23.
In the electrolytic refining of a metal M, what would you take as the anode, the cathode and the electrolyte ?
Cathode – Pure metal
Anode – Impure metal
Electrolyte – Metal salt solution
Question 24.
Pratyush took sulphur powder on a spatula and heated it. He collected the gas evolved by inverting a test tube over it, as shown in the figure.
(a) What will be the action of gas on
(i) dry litmus paper ?
(ii) moist litmus paper ?
(b) Write a balanced chemical equation for the reaction taking place.
(i) Dry litmus paper – no action.
(ii) Moist litmus paper – becomes red.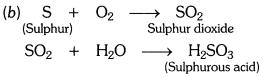
Question 25.
State two ways to prevent the rusting of iron.
Ways to prevent rusting of iron are :
(a) By painting
(b) By galvanizing
Question 26.
What type of oxides are formed when non-metals combine with oxygen ?
Non-metals combine with oxygen to form acidic oxides or neutral oxides.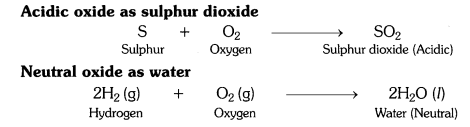
Question 27.
Give reasons :
(a) Platinum, gold and silver are used to make jewellery.
(b) Sodium, potassium and lithium are stored under oil.
(c) Aluminium is a highly reactive metal, yet it is used to make utensils for cooking.
(d) Carbonate and sulphide ores are usually converted into oxides during the process of extraction.
(a) Platinum, gold and silver are used to make jewellery because these are malleable and ductile. These are highly resistant to corrosion.
(b) Sodium, potassium and lithium are very reactive and catch fire when exposed to air. This is due to their low ignition temperature and high reactivity.
(c) Aluminium forms a non-reactive layer of aluminium oxide on its surface. This layer prevents aluminium to react with other substances. That’s why aluminium is used to make cooking utensils.
(d) It is easier to reduce a metal oxide into free metal. Since it is easier to obtain metals from their oxides than from their carbonates or sulphides directly, therefore, the carbonate and sulphide ores are first converted to oxides for extracting the metals.
Question 28.
You must have seen tarnished copper vessels being cleaned with lemon or tamarind juice. Explain why these sour substances are effective in cleaning the vessels.
The sour substances such as lemon or tamarind juice contain acids. These acids dissolve the coating of copper oxide or basic copper carbonate present on the surface of tarnished copper vessels and makes them shining red-brown again.
Question 29.
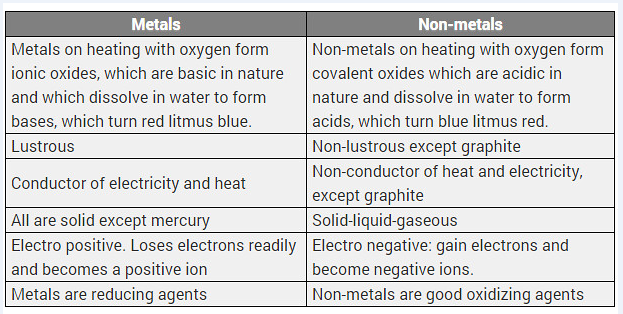
Differentiate between metal and non-metal on the basis of their chemical properties.
Difference between metals and non-metals
| Metals | Non-metals |
| (i) Metals form basic oxides or amphoteric oxides. | (i) Non-metals form acidic or neutral oxides. |
| (ii) Metals replace hydrogen from acids and form salts. | (ii) Non-metals do not replace hydrogen from acids. |
| (iii) With chlorine, metals form chlorides which are electrovalent. | (iii) With chlorine, non-metals form chlorides which are covalent. |
| (iv) With hydrogen few metals form hydrides which are electrovalent. | (iv) With hydrogen, non-metals form many stable hydrides which are covalent. |
Question 30.
A man went door-to door posing as a goldsmith. He promised to bring back the glitter of old and dull gold ornaments. An unsuspecting lady gave a set of gold bangles to him which he dipped in a particular solution. The bangles sparkled like new but their weight was reduced drastically. The lady was upset but after a futile argument the man beat a hasty repeat. Can you play the detective to find out the nature of the solution he has used ?
The dishonest goldsmith dipped the gold bangles in aqua-regia (which contains 1 part of concentrated nitric acid and 3 parts of concentrated hydrochloric acid, by volume). Aqua-regia dissolved a considerable amount of gold from gold bangles and hence reduced their weight drastically. The dishonest goldsmith can recover the dissolved gold from aqua-regia by a suitable treatment.
Question 31.
Give reasons why copper is used to make hot water tanks and not steel (an alloy of iron).
(i) Copper is a better conductor of heat than steel.
(ii) Copper does not corrode easily. But steel corrodes easily.
(iii) Copper does not react with water at any temperature, whereas iron reacts with water on heating.
Question 32
What are amphoteric oxides? Give two examples of amphoteric oxides.
Amphoteric oxides are the oxides, which react with both acids and bases to form salt and water. E.g. ZnO and Al2O3.
Question 33
Name two metals, which will displace hydrogen from dilute acids, and two metals which will not.
Very reactive metals like Zn and Mg displace hydrogen from dilute acids. On the other hand less reactive metals like Cu, Ag, etc. do not displace hydrogen from dilute acids.
Question 34.
In the electrolytic refining of a metal M, what would you take as the anode, the cathode and the electrolyte?
Anode is impure, thick block of metal M.
Cathode is a thin strip/wire of pure metal M.
Electrolyte is a suitable salt solution of metal M.
Question 35.
State two ways to prevent the rusting of iron.
By coating the surface of iron by rust proof paints.
By applying oil or grease to the surface of iron objects so that supply of air consisting of moisture is cut off form the surface.
Question 36
What types of oxides are formed when non-metals combine with oxygen?
When non-metals combine with oxygen it forms either neutral or acidic oxides. CO is a neutral oxide; N2O5 or N2O3 is an acidic oxide.
Question 37.
Give reason
i. Metals replace hydrogen from dilute acids, where as non-metals do not.
ii. Carbonate and sulphide ores are usually converted into oxides during the process of extraction.
i. Metals are electropositive in nature. They readily lose electrons. These electrons reduce the protons liberated from the acid to liberate hydrogen gas, where as non-metals possess a tendency to gain electrons and hence they do not furnish electrons to protons liberated from acids. Hence H2 gas is not liberated.
ii. As it is easier to reduce metal oxides to metal, prior to reduction, metal sulphides and carbonates must be converted to oxides.
Question 38.
Differentiate between metals and non-metals on the basis of their chemical properties.
Question 39.
Explain why the surface of some metals acquires a dull appearance when exposed to air for a long time.
This is due to the surface oxidation of metals when exposed to moist air. For e.g. copper turns green on its surface due to the formation of basic copper carbonate Cu(OH) 2. CuCO3. Similarly silver becomes black due to the formation of black Ag2S and Aluminium forms a white coating of Al2O3 on its surface.
Question 40.
Name a non-metallic element, which conducts electricity.
Carbon in the form of graphite conducts electricity, as there is a free electron in each carbon atom, which moves freely in between the hexagonal layers.
Question 41.
Which metals do not corrode easily?
Gold and platinum and other noble metals do not corrode in air.
Question 12
What are alloys?
Alloys are homogeneous mixtures of two or more metals, or a metal and a non-metal.E.g. steel, brass, bronze, etc.
Question 43
Define the following terms.
(i) Minerals
(ii) Ores
(iii) Gangue
(i) Minerals
All compounds or elements, which occur naturally in the earth’s crust, are called minerals. Example: Alums, K2SO4.Al2(SO4)3 . 24 H2O, Bauxite Al2O3.2H2O
(ii) Ores
Those minerals from which a metal can be profitably extracted are called ores. Bauxite (Al2O3.2H2O) is the ore of Al, copper pyrite CuFeS2. All minerals are not ores but all ores are minerals.
(iii) Gangue
When an ore is mined from the earth, it is always found to be contaminated with sand rocky materials. The impurity of sand and rock materials present in the ore is known as gangue.
Question 44.
Name two metals that are found in nature in the free state.
Gold and platinum are found in the free state in nature.
Question 45
Name two metals, which can form hydrides with metals.
Sodium and calcium form stable hydrides on reacting with hydrogen.
Question 46
Does every mineral have a definite and a fixed composition? Explain.
Yes, every mineral has a definite and a fixed composition. Minerals are widely distributed in the earth’s crust in the form of oxides, carbonates, sulphides, sulphates, nitrates, etc. These minerals are formed as a result of chemical changes taking place during the formation of earth.
Question 47
Explain the meaning of malleable and ductile.
Malleable is being able to be beaten/hammered into thin sheets.
Ductile is being able to be drawn into thin wires.
Question 48
i. Write the electron dot structures for sodium, oxygen and magnesium.
ii. Show the formation of MgO and Na2O by the transfer of electrons.
iii. What are the ions present in these compounds?
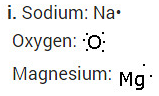
ii. Formation of Magnesium oxide
When magnesium reacts with oxygen, the magnesium atom transfers its two outermost electrons to an oxygen atom. By losing 2 elections, the magnesium atoms form a magnesium ion (Mg2+) and by gaining 2 electrons, the oxygen atom forms an oxide ion (O2-).![]()
Formation of Sodium oxide
Two sodium atoms transfer their 2 outermost electrons to an oxygen atom. By losing two electrons, the two sodium atoms form two sodiumions (2Na+). And by gaining two electrons, the oxygen atom forms an oxide ion (O2-.)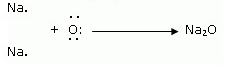
iii. The ions present in sodium oxide compound (Na20) aie sodium ions (2Na+ and oxide ions (O2-).
The ions present in Magnesium oxide compound (MgO) are magnesiumions Mg2+ and oxide ions (O2-).
Question 49
You must have seen tarnished copper vessels being cleaned with lemon or tamarind juice. Explain why these sour substances are effective in cleaning the vessels.
The sour substances such as lemon (or tamarind juice) contain acids. These acids dissolve the coating of copper oxide or basic copper carbonate present on the surface of tarnished copper vessels and make them shining red-brown again.
Question 50.
Give an example of a metal which
i. is a liquid at room temperature.
ii. can be easily cut with a knife.
iii. is the best conductor of heat.
iv. is a poor conductor of heat.
i. Mercury is in liquid state at room temperature.
ii. Sodium and potassium are soft metals which can be easily cut with a knife.
iii. Silver is the best conductor of electricity.
iv. Mercury is a poor conductor of heat.
Question 51
Why is sodium kept immersed in kerosene?.
These compounds are made up of positive and negative ions. There is a strong force of attraction between the oppositively charged ions, so a lot of heat energy is required to break this force of attraction and melt the ionic compounds. This is why ionic compounds have high melting points.
Question 52
A man went door to door posing as a goldsmith. He promised to bring back the glitter of old and dull gold ornaments. An unsuspecting lady gave a set of gold bangles to him which he dipped in a particular solution. The bangles sparkled like new but their weight was reduced drastically. The lady was upset but after a futile argument the man beat a hasty retreat. Can you play the detective to find out the nature of the solution he had used?
Aqua regia (By volume, this contains 3 parts of concentrated hydrochloric acid and 1 part of concentrated nitric acid) is the solution, which is used to sparkle the bangles like new, but their weight will be reduced drastically.
Question 53
Write equations for the reactions of
(i) iron with water
(ii) calcium and potassium with water
Question 54
What would you observe when zinc is added to a sodium of iron(II) sulphate? Write the chemical reaction that takes place?
Zinc is more reactive (more electro positive) than iron. Therefore it displaces iron from its salt solution. The colour of ferrous sulphate is pale green which becomes colourless.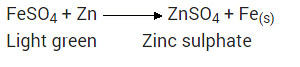
Question 55
Pratyush took sulphur powder on a spatula and heated it. He collected the gas evolved by inverting a test-tube over the burning sulphur.
What will be the action of this gas on:
Dry litmus paper?
Moist litmus paper?
Write a balanced chemical equation for the reaction taking place.
a) When sulphur is brunt in air then sulphur dioxide gas is formed.
(i) Sulphur dioxide gas has no action on dry litmus paper.
(ii) Sulphur dioxide gas turns moist blue litmus paper to red.
(b) S(s) + O2(g) —> SO2(g)
Question 56.
What is the colour of aqueous solution of CuSO4 and FeSO4 as observed in the laboratory?
(a) CuSO4 – blue; FeSO4 – light green
(b) CuSO4 – blue; FeSO4 – dark green
(c) CuSO4 – green; FeSO4 – blue
(d) CuSO4 – green; FeSO4 – colourless
(a) Colour of CuSO4 solution is blue and FeSO4 solution is light green.
Question 57.
A student took four test tubes I, II, III and IV containing aluminium sulphate, copper sulphate? ferrous sulphate and zinc sulphate solutions respectively. He placed an iron strip in each of them.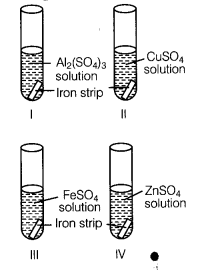
In which test tube, he found a brown deposit?
(a) I
(b) II
(c) III
(d) IV
(b) In test tube II, because Fe is more reactive than copper but less reactive than Al arid Zn..
Question 58.
Aluminium sulphate and copper sulphate solutions were taken in two test tubes I and II respectively. A few pieces of iron filings were then added to both the solutions. The four students A, B, C and D recorded their observations in the form of a table as given below:
| Student | Al2(SO4)3 solution (I) | CuSO4 solution (II) |
| A | Colourless solution -> Light green | Blue colour is retained |
| B | Colourless solution -> No change | Blue colour solution -> Green |
| C | Colourless solution -> Light blue | Blue colour solution -> Green |
| D | No change in colour | Blue colour of solution fades |
Which student has recorded the correct observation?
(a) D
(b) C
(c) B
(d) A
(c) Student B
Iron does not react with Al2(SO4)3 solution because iron is less reactive than aluminium. But Fe being more reactive than Cu displaces Cu from CuSO4 solution.![]()
Question 59.
Aqueous solutions of zinc sulphate and iron sulphate were taken in test tubes I and II by four students A, B, C and D. Metal pieces of iron and zinc were dropped in the two solutions and observations made after several hours were recorded in the form of table as given below:
| Student Solution | Metal | Solution | Colour change Deposit/coating of solution | Deposit/coating obtained |
| A | Fe | ZnSO4 | Turned green | Silvery grey deposit |
| Zn | FeSO4 | No change | No change | |
| B | Fe | ZnSO4 | No change | Black deposit |
| Zn | FeSO4 | Colour faded | Grey coating | |
| C | Fe | ZnSO4 | No change | No change |
| Zn | FeSO4 | Turned colourless | Black deposit | |
| D | Fe | ZnSO4 | No change | Grey deposit |
| Zn | FeSO4 | No change. | Black deposit |
Which student has given the correct report?
(a) B
(b) D
(c) A
(d) C
(d) Student C
(i) Fe is less reactive than zinc. So,![]()
(ii) Zn is more reactive than Fe, so it displaces iron as follows: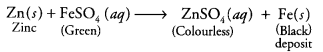
Question 60.
2 mL each of cone. HCl, cone. HNO3 and a mixture of cone. HCl and cone. HNO3 in the ratio of 3 : 1 were taken in test tubes labelled as A, B and C. A small piece of metal was put in each test tube. No change occurred in test tubes A’and Bbut the metal got dissolved in test tube C. The metal could be
(a) Al
(b) Au
(c) Cu
(d) Pt
(b, d) A mixture of cone. HCl and cone. HNO3 in the ratio of 3 : 1 is known as aqua-regia. Gold (Au) and platinum (Pt) dissolve only in aqua-regia as these metals are very less reactive.
Question 61.
When an aluminium strip is kept (a) Green solution of FeSO4 slowly turns brown
(b) Green solution of FeSO4 rapidly turns brown
(c) No change in colour of FeSO4
(d) Green solution of FeSO4 slowly turns colourless
(a) The green solution of ferrous sulphate slowly turns brown. As aluminium is more reactive than iron, it displaces iron from ferrous sulphate solution.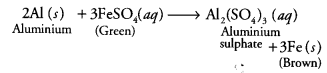
Question 62.
Aluminium is used for making cooking utensils. Which of the following properties of aluminium are responsible for the same?
(i) Good thermal conductivity
(ii) Good electrical conductivity
(iii) Ductility
(iv) Fligh melting point
(a) (i) and (ii)
(b) (i) and (iii)
(c) (ii) and (iii)
(d) (i) and (iv)
(d) Good thermal conductivity, malleability, light weight and high melting point are the properties/of aluminium due to which it-is used for making cooking utensils.
Question 63.
If copper is kept open in air, it slowly loses its shining brown surface and gains a green coating. It is due to the formation of
(a) CuSO4
(b) CuCO3
(c) CU(NO3)2
(d) CuO
(b) Copper reacts with CO2 present in air and forms a green coating on its surface due to the formation of basic copper carbonate [CuCO3.Cu(OH)2] as: