Q 1 – The metal which is liquid at room temperature is
(a) sodium
(b) bromine
(c) calcium
(d) mercury
Q 2 – Name the gas evolved when magnesium reacts with dilute hydrochloric acid
(a) Chlorine
(b) Oxygen
(c) hydrogen
(d) Nitrogen
Q 3 – The best electrical conductor is
(a) gold
(b) copper
(c) silver
(d) aluminium
Q 4 – Non-metals are
(a) non-ductile
(b) non-sonorous
(c) non-malleable
(d) all of these
Q 5 – Metalloids possess the properties of
(a) metals
(b) non-metals
(c) both metals and non-metals
(d) none of these
Q 6 – Boron is
(a) metal
(b) metalloid
(c) non-metal
(d) alkali
Q 7 – A mineral from which. a metal can be extracted on the commercial scale, economically is called
(a) ore
(b) metalloid
(e) corrosion
(d) metal
Q 8 – Which of the following metal is stored in kerosene?
(a) Sodium
(b) Magnesium
(c) Phosphorus
(d) Zinc
Q 9 – Which metal is used in wrapping materials?
(a) Aluminium
(b) Zinc
(c) Copper
(d) None of these
Q 10 – Iron is galvanised by coating it with
(a) chromium
(b) sodium
(c) magnesium
(d) zinc
Q 11 – Out of these, which one is more reactive with water?
(a) Sodium
(b) Magnesium
(c) Iron
(d) Copper
Q 12 – Materials having qualities of both metals and non-metals are
(a) alloys
(b) metalloids
(c) noble metals
(d) none of these
Q 13 – Which metal reacts readily with cold water?
(a) Gold
(b) Silver
(c) Magnesium
(d) Calcium
Q 14 – The metal which is not corroded by air, water and acid is
(a) copper
(b) zinc
(c) aluminium
(d) gold
Q 15 – Metals are
(a) soft and brittle
(b) hard and solid
(c) liquid
(d) generally liquid
Q 16 – Which one of the following metals is the most reactive and stored in kerosene?
(a) Iron
(b) Gold
(c) Copper
(d) Potassium
Q 17 – Which of the following metals are soft enough to be even cut with a knife?
(a) Sodium
(b) Potassium
(c) Lithium
(d) All of these
Q 18 – The most reactive metal is
(a) copper
(b) silver
(c) potassium
(d) calcium
Q 19 – Which one of the following metals is the most ductile?
(a) Aluminium
(b) Copper
(c) Silver
(d) Gold
Q 20 – Which of the following non-metals are used in fertilisers?
(a) Nitrogen
(b) Phosphorus
(c) Both (a) and (b)
(d) None of these
Q 21 – Which one of the following is a metal?
(a) C
(b) N
(c) Na
(d) O
Q 22 – What happens when magnesium is burnt in air?
Q 23 – Name some common non-metals.
Q 24 – Name two metals which are soft enough to be cut.
Q 25 – Zinc sulphate forms a colourless solution in water. Will you observe any colour on adding copper turn-ing in it?
Q 26 – Why does calcium float in water?
Q 27 – What is a metal?
Q 28 – What are non-metals?
Q 29 – Explain the term ‘malleability’ with suitable examples.
Q 30 – Why aluminium is used for wrapping of food items?
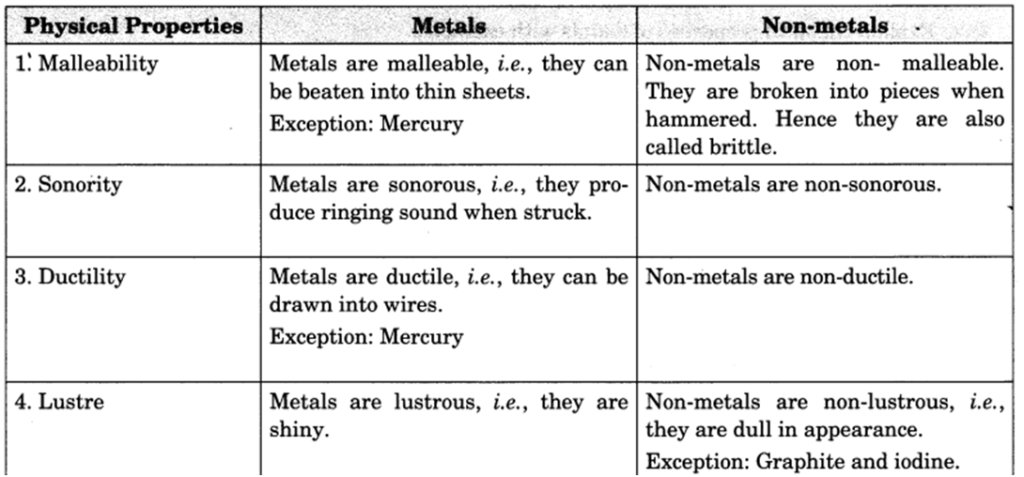
Q 31 – What are the differences between metals and non-metals? Explain on the basis of their physical properties.
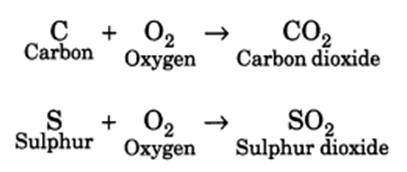
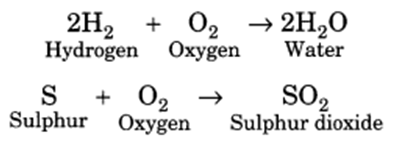
Q 32 – With the help of equations, explain the reaction of non-metals with oxygen.
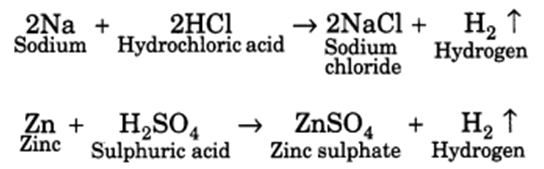
Q 33 – How do metals and non-metals react with acids?
Q 34 – Distinguish between metals and non-metals on the basis of their physical properties.
Q 35 – Explain with suitable examples the chemical properties of non-metals.