Q 1. Which postulate of Dalton’s Atomic theory is the basis of law of conservation of mass ?
Ans. The law of conservation mass is based on the following postulate of Dalton’s Atomic theory. “Atoms can neither be created nor destroyed during a physical change or a chemical reaction.”
Q 2. Which postulate of Dalton’s Atomic theory can explain the law of definite proportions ?
Ans. The law of definite proportions is based on the following postulate of Dalton’s Atomic theory.
“All atoms of a particular element are identical in every respect. This means that they have same mass, same size and also same chemical properties.”
Q 3. Define atomic mass unit.
Ans. Atomic mass unit may be defined as :
The mass of one-twelfth (1/12) of the mass of one atom of carbon taken as 12 u. It is represented as 1 u (unified mass).
Q 4. Why is not possible to see an atom with naked eye ?
Ans. It is not possible to see an atom with naked eye because of its extremely small size. For example, the radius of an atom of hydrogen is of the order of 10-10 m. Actually an atom is regarded as a microscopic particle. These microscopic particles cannot be seen with naked eye.
Q 5. What is meant by the term chemical formula ?
Ans. Molecule represents a group of two or more atoms (same or different) chemically bonded to each other and held tightly by strong attractive forces. Molecules are represented in terms of symbols of constituting atoms and it is known as chemical formula.
Q 6. Out of atoms and molecules, which can exist independently ?
Ans. Molecules can exist independently. However, the atoms of noble gases (He, Ne, Ar, Kr, Xe) can also exist independently.
Q 7. What does the symbol ‘u’ represent ?
Ans. The symbol V represents unified mass.
Q 8. Write the chemical symbols and Latin names of
(i) gold
(ii) mercury
Ans.
(i) Au (Aurum)
(ii) Hg (Hydrargyrum).
Q 9. Are the mass of a molecule of a substance and its molar mass same ?
Ans. No, they are different.
Q 10. Avogardo’s number represents how many particles ?
Ans. Avogadro’s number (Ng) represents 6.022 x 1023 particles.
Q 11. What happens to an element ‘A’ if its atom gains two electrons ?
Ans. It changes to a divalent anion (A2-).
Q 12. Why is a cation so named ?
Ans. When electric current is passed through the solution of a salt like sodium chloride (NaCl), the positive ion (Na+) migrates towards cathode (negative electrode). It is therefore, called cation. Please remember that
• Positive ion migrating towards cathode on passing electric current is known as cation.
• Negative ion migrating towards anode on passing electric current is known as anion.
Q 13. An element Z forms an oxide with formula Z2O3. What is its valency ?
Ans. The valency of the element Z in Z2O3 is 3+.
Q 14. The valency of an element A is 4. Write the formula of its oxide.
Ans. The formula of the oxide is A2O4 or AO2.
Q 15. An element X has valency 3 while the element Y has valency 2. Write the formula of the compound between X and Y.
Ans. he formula of the compound between X and Y is X2Y3.
Q 16. Formula of the carbonate of a metal M is M2CO3. Write the fomula of its chloride.
Ans. The valency of the metal (M) in M2CO3 is (1+) i.e. metal exists as M+ ion. Therefore, the formula of metal chloride is MCl.
Q 17. What do you understand from the statement “relative atomic mass of sulphur is 32”.
Ans. This means that an atom of sulphur is 32 times heavier as compared to 1/12 of the mass of 1 atom of C — 12(1 u)
Q 18. Calculate the formula unit mass of CaCl2.
Ans. Formula unit mass of CaCl2 (Calcium chloride)
= (1 x Atomic mass of Ca) + (2 x Atomic mass of Cl)
= (1 x 40 u) + (2 x 35.5 u) = 111 u
Q 19. Which of the following represents the correct chemical formula ?
(a) NaSO4
(b) CaPO4
(c) ZnS
(d) AlSO4
Ans. The formula (c) represents the correct formula. Both the ions are divalent i.e. Zn2+ and S2-. The name of the compound is zinc sulphide.
Q 20. Sample A contains one gram molecules of oxygen molecules and sample B contains one mole of oxygen molecules. What is the ratio of the number of molecules in both the sample ?
Ans. One gram molecules and one gram mole contain the same number of molecules (6.022 x 1023). Therefore, the ratio is 1 : 1.
Q 21. Gram molecular mass of ammonia (NH3) is 17 g. Is it correct to regard it as formula unit mass also ?
Ans. No, it is not correct. Ammonia exists in molecular form and is not an ionic compound made up of cation and anion. Therefore, it cannot have formula unit mass. It has only molecular mass.
Q 22. Give one example each of polyatomic element and polyatomic ion.
Ans. Polyatomic element (P4) ; Polyatomic ion (SO42-).
Q 23. Name the element which is used as a reference for the atomic masses of the elements.
Ans. 1/12 of the mass of carbon atom taken as 12 u is used as a reference for the atomic masses of the elements.
Q 24. Four samples of water [H2O] are collected from different sources. Each sample on analysis was found to contain same percentage of oxygen. Which law of chemical combination is demonstrated by the above observation ?
Ans. The law of constant combination is demonstrated by this observation.
Q 25. Calculate the molar mass of ethyl alcohol (C2H5OH).
Ans. Molar mass of C2H5OH= (2 x gram atomic mass of C) + (6 x gram atomic mass of H)
+ (1 x Atomic mass of O)
= (2 x 12 g) + (6 x 1 g) + (1 x 16 g) = 46 g.
Q 26. Write the chemical symbols of two elements ?
(a) Which are formed from the first letter of the elements name.
(b) Whose name has been taken from the names of the elements in Latin.
Ans.
(a) Boron (B), carbon (C)
(b) Argentum (Ag), Kalium (K).
Q 27 . (a) If the valency of carbon is 4 and that of sulphur is 2, write the formula of the compound formed between carbon and sulphur atoms. Also name the compound.
(b) What is wrong with the statement T mole of hydrogen ?
Ans.
(a) The formula of compound can be written by exchanging the valencies (cross-over). Therefore, the expected formula is C2S4 or CS2. The compound is called carbon disulphide.
(b) The statement is not correct. We must always write whether hydrogen is in atomic form or molecular form. The correct statement is :
Q 28. T mole of hydrogen atoms or one mole of hydrogen molecules.
Identify the cations and anions in the following compounds :
(a) CH3COONa
(b) NH3
(c) NH4Cl
(d) SrCl2.
Ans.
(a) CH3COO–, Na+
(b) It is a molecular compound
(c) NH4+ ,Cl– (d) Sr2+, 2Cl–
Q 29. Classify the following based on atomicity
(a) O3
(b) P4
(c) S8.
Ans.
Atomicity is the number of atoms present in one molecule of an element. Based upon this, the atomicity of different molecules may be expressed as :
(a) 3
(b) 4
(c) 8.
Q 30. Give the formulae of the compounds that will be formed from the following sets of elements.
(a) Calcium and fluorine
(b) Magnesium and oxygen
(c) Sodium and sulphur
(d) Carbon and chlorine
(e) Carbon and sulphur
(f) Nitrogen and hydrogen
Ans.
(a) CaF2
(b) MgO
(c) Na2S
(d) CCl4
(e) CS2
(f) NH3.
Q 31. Which of the following symbols of elements are incorrect. Write correct symbols
(a) Iron (Ir)
(b) Gold (Au)
(c) Manganese (M)
(d) Potassium (Po)
(e) Zinc (ZN)
(f) Calcium (Ca).
Ans.
(a) Iron (Fe)
(c) Manganese (Mn)
(d) Potassium (K)
(e) Zinc (Zn).
Q 32. Verify by calculation that :
4 moles of CO2 and 6 moles of H2O do not have same mass in grams
Ans.
Molar mass of CO2 = 12 + 2 x 16 = 44 g
1 mole of CO2 has mass = 44 g
4 moles of CO2
have mass = 44 x 4 = 176 g
Molar mass of H2O = 2 x 1 + 16 =18 g
1 mole of H2O has mass = 18 g
6 moles of H2O have mass = 6 x 18 = 108 g.
This shows that these have different masses in grams.
Q 33. A sample of vitamin C contains 2.48 x 1025 oxygen atoms. How many moles of oxygen atoms are present in the sample ?
Ans.

Q 34. Calculate the total number of ions in 0.585 g of sodium chloride.
Ans.
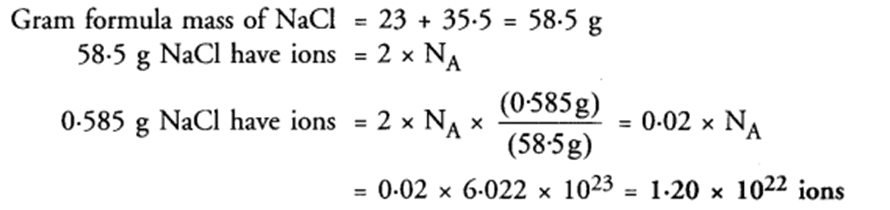
Q 35. A flask contains 4.4 g of CO2 gas. Calculate
(a) How many moles of CO2 gas does it contain ?
(b) How many molecules of CO2 gas are present in the sample.
(c) How many atoms of oxygen are present in the given sample.
(Atomic mass of C = 12 u. O = 16 u)
Ans.

Q 36. Determine the molecular mass of :
(i) NH4OH
(ii) K2CO3
(iii) CH3COOH Given atomic masses :
H = 1.0 u ; O = 16.0 u ; C = 12.0 ; K = 39.0 u ; N = 14.0 u
Ans.
(i) Molecular mass of NH4OH
= Atomic mass of N + 5 x (Atomic mass of H) + Atomic mass of O
= 14 + 5 x 1 + 16 = 14 + 5 + 16 = 35 u
(ii) Molecular mass of K2CO3
= 2 x (Atomic mass of K) + Atomic mass of C + 3 x (Atomic mass of O)
= 2 x 39 + 12 + 3 x 16 = 138 u
(iii) Molecular mass of CH3COOH
= 2 x (Atomic mass of C) + 4 x (Atomic mass of H) + 2 x (Atomic mass of O)
= 2 x 12 + 4 x 1+2 x 16 = 60 u
Q 37. (a) What is Avogadro Constant ?
(b) Calculate the number of particles present in 56 g of N2 molecule.
Ans.(a) Avogadro’s number is the number of particles (atoms, ions, molecules etc.) present in one mole of any substance. It is denoted either as NA or as NQ. The number is also called Avogadro’s constant because its value is fixed (6.022 x 1023) irrespective of the nature of the particles.
(b) Molar mass of N2 = 14 x 2 = 28 g
28 g of N2 have molecules or particles = NA = 6.022 x 1023
56 g of N2 have molecules or particles = NA = 6.022 x 1023 x 2 = 1.204 x 1024
Q 38. (a) An element ‘X’ exhibits variable valencies 3 and 5. Write the formulae of the chlorides of the element.
(b) What is the ratio by mass of the elements present in the chemical formula of magnesium oxide
Ans.
(a) The formulae of the chlorides of the element ‘X’ = XCl5 and XCl3.
(b) The chemical formula of magnesium oxide is MgO. The elements are present in the ratio of 24 : 16 or 3 :2
Q 39. Give the example of trivalent cation and monovalent anion. Write the formula of the compound formed by their combination. .
Ans. Trivalent cation : Al3+ ; Monovalent anion : Cl–
Formula of the compound : AlCl3.
Q 40. (a) State the law of conservation of mass
(b) What mass of silver nitrate will react with 5.85 g of sodium chloride to produce 14.35 g of silver chloride and 8.5 g of sodium nitrate if the law of conservation of mass is true ?
Ans.
(a) The total mass of the products in a physical change or a chemical reaction is equal to the total mass of the reactants that have combined. The law may also be stated in another form. The mass can neither he created nor destroyed in a physical change or a chemical reaction.
(b) The chemical reaction leading to products is :
Mass of reactants = x g + 5.85 g Mass of products = 14.35 g + 8.5 g According to law of conservation of mass
Mass of reactants = Mass of products . (x + 5.85) g = (14.35 + 8.5) g
x = (22.85 – 5.85) g = 17.0 g
Q 41. (a) The mass of one molecule of a substance is 4.65 x 10-23 g. What is its molecular mass ? What could this substance be ?
(b) Which have more molecules? 10 g of sulphur dioxide (SO2) or 10 g of oxygen (O2) ?
Ans.

Q 42. Which has more atoms ?
(a) 10 g of nitrogen (N2)
(b) 10 g of ammonia (NH3)
Ans.
Q 43. (a) Give one point of difference between an atom and an ion.
(b) Give one example each of a polyatomic cation and a polyatomic anion.
(c) Identify the correct chemical name of FeSO4 from the given names Ferrous sulphate, Ferrous sulphide, Ferrous sulphite.
(d) Write the chemical formula for the chloride of magnesium.
Ans.

(a) An atom is always neutral in the sense that it does not carry any charge. An ion carries either positive charge (cation) or negative charge (anion).
(b) Ammonium (NH4)+ ion is a polyatomic cation while sulphate (SO4)2- ion is a polyatomic anion.
(c) Correct chemical name of FeSO4 is ferrous sulphite.
(d) Chemical formula for the chloride of magnesium is MgCl2.
Q 44. (a) What do the following observations stand for ?
(i) 2O
(ii) 3O2
(b) Which amongst the following has more number of atoms and how much ?
(i) 11.5 g of sodium
(ii) 15.0 g of calcium
Ans. (a) (i) 2O represent two atoms of oxygen (or the gram atoms of oxygen
(ii) 3O2 represent three molecules of oxygen or three gram moles of oxygen.
Q 45. Hydrogen and oxygen combine in the ratio of 1:8 by mass to form water. What mass of
oxygen gas would be required to react completely with 3 g of hydrogen gas?
Ans. As per the given 1:8 ratio mass of oxygen gas required to react completely with 1g of
hydrogen gas is 8g.
Therefore mass of oxygen gas required to react completely with 3g of hydrogen gas will be = 3 X 8 = 24g
Q 46. Which postulate of Dalton’s atomic theory is the result of the law of conservation of
mass?
Ans. The postulate of Dalton’s atomic theory which is the result of the law of conservation of mass is mentioned as below :
Atoms are indivisible particles, which cannot be created or destroyed in a chemical reaction.
Q 47. Which postulate of Dalton’s atomic theory can explain the law of definite proportions?
Ans. The postulate of Dalton’s atomic theory which explains the law of definite proportions is
“Atoms combine in the ratio of small whole numbers to form compounds and the relative
number and kinds of atoms are constant in a given compound.”
Q 48. Why is it not possible to see anatom with naked eyes?
Ans. An atom is an extremely minute particle and as such actual mass of an atom of hydrogen is
considered to be 1.6X10-24 g. That is why it is not possible to see an atom with naked eyes.
Q 49. Which has more number of atoms, 100 grams of sodium or100 grams of iron (given,
atomic mass of Na = 23 u, Fe = 56 u)?
Ans. We can find out the element with more number of atoms by calculating number of moles
of each of them :
Number of moles of sodium in 100g = m1/M1 = 100/23 = 4.34
Number of moles of iron in 100g = m2/M2 = 100/56 = 1.79
Therefore, the number of atoms is more for sodium as compared to iron.
Q 50. When 3.0 g of carbon is burnt in 8.00 g oxygen, 11.00 g of carbon dioxide is produced.
What mass of carbon dioxide will be formed when 3.00 g of carbon is burnt in 50.00 g of
oxygen? Which law of chemical combination will govern your answer?
Ans. According to the law of chemical combination of constant proportions “in a chemical
compound the elementary constituents always combine in constant proportions by
weight/mass”. Therefore whether 3 g carbon is burnt in 8 g oxygen or 3g carbon is burnt in 50g oxygen in both cases only 11g carbon dioxide will be formed
Q 51. Define Valency? Find the Valency of oxygen and Aluminum.
Ans. Valency is defined as the number of electrons that an element has to gain or loose from its outermost shell so that it can be stable or the combining capacity of an atom.
Oxygen – Atomic number = 8; Electronic configuration = 2, 6 i.e. it has to gain 2e- so that in :
outer most shell has 8e-, Valency of O is -2 Similarly, valency of Al (Aluminum) is +3 ( electronic configuration → 2, 8, 3) so it looses 3e- from its outermost shell.
Q 52. State the law of constant Proportion?
Ans. According to law of constant proportion, whatever the method of its formation, a chemical compound in its pure state will always contain the same elements combined together in the fixed ratio by mass.
Q 53. Define the terms:- a) Atomic number b) Mass number
Ans. Atomic Number is defined as the total number of protons present in an atom.
Mass number is defined as the sum total of number of protons and the number of neutrons present in an atom
Q 54. What is meant by the term chemical formula?
Ans. A chemical formula is the representation of elements present in a compound with the help of symbols and also the number of atoms of each element with those numbers only. For e.g.: A molecule of water (compound) contains 2 atoms of hydrogen and one atom of oxygen hence itschemical formula is H2O.
Q 55. Which element will be more reactive and why → the element whose atomic number is
10 or the one whose atomic number is 11?
Ans. Element with atomic number 11 is more reactive than the one with atomic number 10 because electronic configuration of atomic number 11 will be 2, 8, 1 so, it has to loose only 1efrom its outermost shall to be stable which is more easy than the element with atomic number 10 because its electronic configuration is 2, 8 and has 8e- in the outermost shell and hence is already stable.
Q 56. (a) Four samples of carbon dioxide (CO2) were prepared by using different methods. Each sample on analysis was found to contain 27.27% carbon by mass. Name the law which is in agreement with this observation.
(b) Explain why the number of atoms in one mole of hydrogen gas is double the number of atoms in one mole of helium gas.
Ans. (a) Carbon dioxide consists of elements carbon and oxygen. Since the percentage of carbon in each sample is fixed, that of oxygen must be also fixed. This is according to law of constant proportions.
(b) Hydrogen gas is diatomic in nature (H2) while helium gas is monoatomic (He). As a result, the number of atoms in one mole of hydrogen. (2 x NA) are expected to be double as compared to number of atoms in one mole of helium (NA).