Work done on an object is defined as the product of the magnitude of the force acting on the body and the displacement in the direction of the force. W = F.s. The SI unit of force is Newton.
If a force acting on a body causes no displacement, the work done is 0. For example, pushing a wall.

The force component F cos θ gives the component of force along the direction that the body is displaced. Cos θ is the angle between the force vector and displacement vector.
Defining Work
The scientific definition of work is different in many ways from its everyday meaning. The definition of work in physics reveals its relationship to energy – whenever work is done, energy is transferred.
For work to be done, in a scientific sense, a force must be exerted and there must be displacement in the direction of the force. With this said, we can say that
Work is the product of the component of the force in the direction of the displacement and the magnitude of this displacement.
Mathematically, the above statement is expressed as follows:
W = (F cos θ) d = F. d
Where,
- W is the work done by the force.
- F is the force, d is the displacement caused by the force
- θ is the angle between the force vector and the displacement vector
The dimension of work is the same as that of energy and is given as, [ML2T–2].
Unit of Work
The SI unit of work is the joule (J), which is defined as the work done by a force of 1 Newton in moving an object through a distance of 1 meter in the direction of the force.

The work done upon the weight against gravity can be calculated as follows:
Work Done = (Mass × acceleration due to gravity) × Displacement
= (25 × 9.8) × 2 J
Factors Affecting Work
Let us now consider the factors on which work done on an object by a force depends.
Force:
Force is defined as a push or a pull that can cause any object with a mass to change its velocity and acceleration. Force is a vector quantity and has both a magnitude and a direction. If the force acting on an object is zero irrespective of the state of the object (dynamic or static) that work done by the force is zero.
Displacement:
Displacement is a vector quantity that gives the shortest distance between the initial position and the final position of any object. If the resulting displacement in the direction of force, due to force acting on any object is zero, the net work done by that force on that object is zero. For e.g., if we push a rigid wall with all our might and still fail to displace it, then we can say no work has been performed by us on the wall.
The Angle between the Force Vector and the Displacement Vector

The work done by a force on an object can be positive, negative, or zero, depending upon the direction of displacement of the object with respect to the force. For an object moving in the opposite direction to the direction of force, such as friction acting on an object moving in the forward direction, the work is done due to the force of friction being negative.
Similarly, an object experiences a zero force when the angle of displacement is perpendicular to the direction of the force. Consider an example of a coolie lifting a mass on his head moving at an angle of 90˚ with respect to the force of gravity. Here, the work done by gravity on the object is zero.
Energy
Energy is defined as the ability to do work. Its unit is the same as that of work. Energy is a scalar quantity.
SI unit of energy or work = Joule (Nm) or Kgm2s−2.
Forms of Energy
Energy has different forms: Light, heat, chemical, electrical or mechanical.
Mechanical energy is the sum of:
(i) Kinetic energy (K.E)
(ii) Potential energy (P.E)
What is Energy
There are different forms of energy on this planet. The Sun is considered the elemental form of energy on the Earth. In Physics, energy is considered a quantitative property that can be transferred from an object in order for it to perform work. Hence, we can define energy as the strength to do any kind of physical activity. Thus, they say,
Energy is the ability to do work
According to the laws of conservation of energy, it states that “the energy can neither be created nor destroyed but can only be converted from one form to another”. The SI unit of energy is Joule. In this article, let us understand in detail about units of energy and different forms of energy.
Units of Energy
The International System of Units of measurement of energy is Joule. The unit of energy is named after James Prescott Joule. Joule is a derived unit and it is equal to the energy expended in applying a force of one newton through a distance of one meter. However, energy is also expressed in many other units not part of the SI, such as ergs, calories, British Thermal Units, kilowatt-hours, and kilocalories, which require a conversion factor when expressed in SI units.
Energy Conversion: Transfer and Transform
We know that energy can be transferred from one form to another, the movement of energy from one location to another is known as energy transfer. We notice various energy transformations happening around us.
Following are the four ways through which energy can be transferred:
- Mechanically – By the action of force
- Electrically – Electrically
- By Radiation – By Light waves or Sound waves
- By Heating – By conduction, convection, or radiation
The process which results in the energy changing from one form to another is known as energy transformation. While energy can be transformed or transferred, the total amount of energy does not change – this is called energy conservation.
Law of Conservation of Energy
The law of conservation of energy is one of the basic laws in physics. It governs the microscopic motion of individual atoms in a chemical reaction. The law of conservation of energy states that “In a closed system, i.e., a system that is isolated from its surroundings, the total energy of the system is conserved.” According to the law, the total energy in a system is conserved even though the transformation of energy occurs. Energy can neither be created nor destroyed, it can only be converted from one form to another.
Different Types of Energy

Although there are many forms of energy, it is broadly categorized into:
- Kinetic Energy
- Potential Energy
Although there are many forms of energy, it is broadly categorized into:
- Kinetic Energy
- Potential Energy
Kinetic Energy
Kinetic energy is the energy associated with the object’s motion. Objects in motion are capable of causing a change or are capable of doing work. To better understand, let us think of a wrecking ball. A wrecking ball in motion is used to do work such as the demolition of buildings, stones, etc. Even a slow-moving wrecking ball is capable of causing a lot of damage to another object such as an empty house. However, a wrecking ball that is not in motion, does not do any work.
Another example of kinetic energy is the energy associated with the constant, random bouncing of atoms or molecules. This is also known as thermal energy. The average thermal energy of a group of molecules is what we call temperature, and when thermal energy is being transferred between two objects, it’s known as heat.
Kinetic energy is determined by the given formula

Different Types of Kinetic Energy:
Radiant energy
- Radiant energy refers to the type of energy that travels by waves or particles. This energy is created through electromagnetic waves and is most commonly experienced by humans in the form of heat. Following are a few examples of radiant energy:
- When you turn on an incandescent light bulb, it gives off two forms of energy. There is visible light and heat that are generated. Both these generated energies are a form of radiant energy.
- Sunlight is an example of radiant energy.
Thermal Energy
Thermal energy is similar to radiant energy and is experienced in the form of heat or warmth. While radiant energy refers to waves or particles, thermal energy describes the level of activity among the atoms and molecules in an object. This is the only difference between radiant energy and thermal energy. Some examples of thermal energy include:
- The geothermal energy that comes from the decay of natural minerals and the volcanic action of the earth is an example of thermal energy.
- When you heat up the pizza in the oven, you are raising the temperature of the pizza. The molecules that make up the pizza are moving more quickly when the pizza is piping hot.
- The warmth that you feel emanating from the engine is an example of thermal energy.
Sound Energy
The vibrations that reach the human ear are experienced by humans as sound. The disturbance moves in the form of waves through a medium-like air and reaches our eardrum. On reaching the eardrum, these vibrations are converted into electrical signals and sent to the brain which we interpret as the sensation of sound.
Electrical Energy
The flow of negatively charged electrons around a circuit results in electricity which we more commonly referred to as electrical energy.
Mechanical Energy
Mechanical energy is the energy associated with the mechanical movement of objects.
Potential Energy
Potential energy is the energy stored in an object or system of objects. Potential energy has the ability to transform into a more obvious form of kinetic energy.
Potential energy is determined by the given formula
Potential energy = m ×g ×h
Both potential energy and kinetic energy form mechanical energy.
Mechanical energy is determined by the following formula
Mechanical energy = 1/(2)mv2+mgh
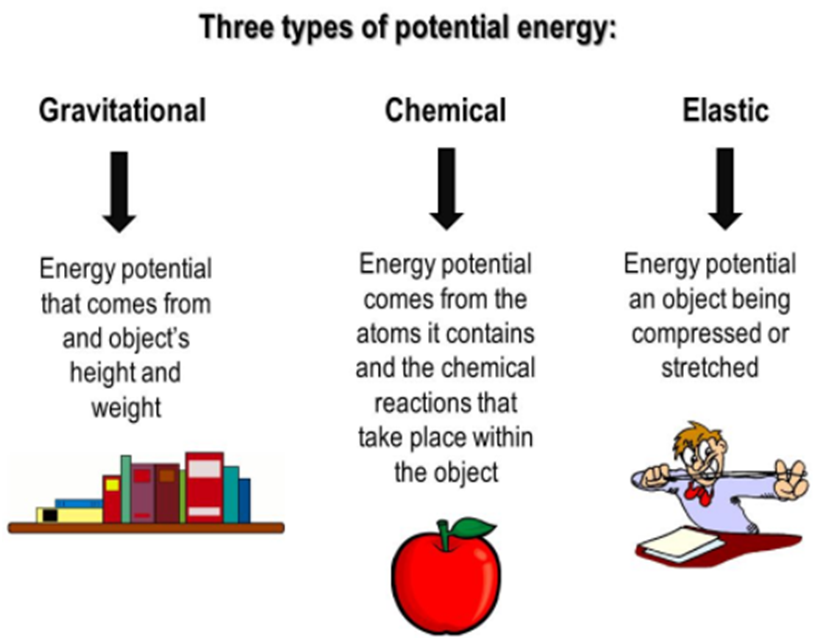
Different Types Of Potential Energy
Gravitational Potential Energy
Gravitational potential energy is the energy stored in an object as the result of its vertical position or height. A book on a high bookshelf has a higher gravitational potential energy than the book at the bottom bookshelf.
Elastic Potential Energy
Elastic potential energy is energy stored as a result of applying a force to deform an elastic object. The energy is stored until the force is removed and the object springs back to its original shape, doing work in the process. The deformation could involve compressing, stretching or twisting the object.
Chemical Potential Energy
Chemical potential energy is the energy stored in the chemical bonds of the substance. It is the energy that can be absorbed and released due to a change in the particle number of the given species.
Electric Potential Energy
Electric potential energy is the energy that is needed to move a charge against an electric field.
Some of the examples of electric potential energy include:
- An incandescent light bulb that is turned off
- A radio tower that is not working
- A black light turned off
- A television before it is turned on

Although there are many forms of energy, it is broadly categorized into:
- Kinetic Energy
- Potential Energy
Kinetic Energy
Kinetic energy is the energy associated with the object’s motion. Objects in motion are capable of causing a change or are capable of doing work. To better understand, let us think of a wrecking ball. A wrecking ball in motion is used to do work such athe s demolition of buildings, stones, etc. Even a slow-moving wrecking ball is capable of causing a lot of damage to another object such as an empty house. However, a wrecking ball that is not in motion, does not do any work.
Another example of kinetic energy is the energy associated with the constant, random bouncing of atoms or molecules. This is also known as thermal energy. The average thermal energy of a group of molecules is what we call temperature, and when thermal energy is being transferred between two objects, it’s known as heat.
Kinetic Energy
Objects in motion possess energy and can do work. This energy is called Kinetic Energy.
F=ma
Also,
W=F
From the second equation of motion, we know that

Rearranging the equation, we get

Substituting equation for work done by a moving body,
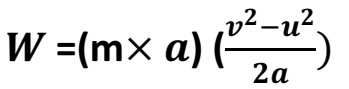
Taking intial velocity as zero, we get

When two identical bodies are in motion, the body with a higher velocity has more K.E.

Types of Kinetic Energy
There are five types of kinetic energy: radiant, thermal, sound, electrical and mechanical. Let us look at some of the kinetic energy examples and learn more about the different types of kinetic energy.
Radiant energy is a type of kinetic energy that is always in motion travelling through medium or space. Examples of radiant energy are:
- Ultraviolet light
- Gamma rays
Thermal energy
Thermal energy, known as heat energy, is generated due to the motion of atoms when they collide with each other. Examples of thermal energy are:
- Hot springs
- Heated swimming pools
Sound energy
The vibration of an object produces sound energy. Sound energy travels through the medium but cannot travel in a vacuum as there are no particles to act as a medium. Examples of sound energy are:
- Tuning fork
- Beating drums
Electrical energy
Electrical energy is obtained from the free electrons that are of positive and negative charge. Examples of electrical energy are:
- Lightning
- Batteries when in use
‘Mechanical energy
The sum of kinetic energy and potential energy is known as mechanical energy, which can neither be created nor be destroyed but converted from one form to another. Examples of mechanical energy are:
- Orbiting of satellites around the earth
- A moving car
- Difference Between Kinetic Energy and Potential Energy
| Kinetic energy | Potential energy |
| Kinetic energy is a form of energy possessed by an object due to its motion. | Potential energy is the form of energy possessed by an object due to its position or state. |
| Formula used is | The formula used is mgh |
| Vibrational energy is an example of kinetic energy | Gravitational potential energy is an example of potential energy |
Factors affecting kinetic energy
- Mass
- Velocity
- Momentum
Potential Energy
Energy can get stored in an object when work is done on it.
For example, stretching a rubber string. The energy that is possessed by a body by virtue of its configuration or change in position is known as Potential Energy.
The potential energy of an object at a height.
When an object is raised to a certain height, work is done against gravity to change its position. This energy is stored as Potential Energy.
⇒W = F.s
⇒F = ma
In the case of increasing the height, F = mg
Therefore , W (P.E) = mgh
⇒ ΔPE=mg(h final−h initial)
Law of Conservation of Energy
The Law of conservation of energy states that energy can neither be created nor destroyed, but can be transferred from one form to another. The total energy before and after the transformation remains constant.
Total energy = KE + PE
where, 1/2 mv2 + mgh = constant
For example: consider a ball falling freely from a height. At height h, it has only PE = mg h.
By the time it is about to hit the ground, it has a velocity and therefore has KE= 1/2
mv2. Therefore, energy gets transferred from PE to KE, while the total energy remains the same.
What is the Law of Conservation of Energy?
The law of conservation of energy states that energy can neither be created nor be destroyed. Although, it may be transformed from one form to another. If you take all forms of energy into account, the total energy of an isolated system always remains constant. All the forms of energy follow the law of conservation of energy. In brief, the law of conservation of energy states that
In a closed system, i.e., a system that is isolated from its surroundings, the total energy of the system is conserved.
So in an isolated system such as the universe, if there is a loss of energy in some part of it, there must be a gain of an equal amount of energy in some other part of the universe. Although this principle cannot be proved, there is no known example of a violation of the principle of conservation of energy.
The amount of energy in any system is determined by the following equation:

- UT is the total energy of a system
- Ui is the initial energy of a system
- Q is the heat added or removed from the system
- W is the work done by or on the system
The change in the internal energy of the system is determined using the equation

Energy Conservation:
Energy conservation is not about limiting the use of resources that will finally run out altogether. The ideal way of conservation would be reducing demand on a limited supply and enabling that supply to begin to rebuild itself. Many times the best way of doing this is to replace the energy used with an alternative.
Law of Conservation of Energy Examples:
In Physics, most inventions rely on the fact that energy is conserved when it is transferred from one form to another. A number of electrical and mechanical devices operate solely on the law of conservation of energy. We will discuss a few examples here.
- In a torch, the chemical energy of the batteries is converted into electrical energy, which is converted into light and heat energy.
- In hydroelectric power plants, waterfalls on the turbines from a height. This, in turn, rotates the turbines and generates electricity. Hence, the potential energy of water is converted into the kinetic energy of the turbine, which is further converted into electrical energy.
- In a loudspeaker, electrical energy is converted into sound energy.
- In a microphone, sound energy is converted into electrical energy.
- In a generator, mechanical energy is converted into electrical energy.
- When fuels are burnt, chemical energy is converted into heat and light energy.
- Chemical energy from food is converted to thermal energy when it is broken down in the body and is used to keep it warm.
Power
The rate of doing work or the rate of transfer of energy is called power. It is denoted by P
⇒ P =

SI unit is Watt (Js−1).
Average power = Total energy consumed/Total time taken
Commercial Unit of Power
The commercial unit of power is kWh i.e. energy used in 1 hour at 1000 Joules/second.
1kWh=3.6×106J