We, at cbseinsights.com, have come up with the latest Acids-Bases and Salts For Class-10 Chemistry-MCQ as per the latest CBSE notification for 2021-22.
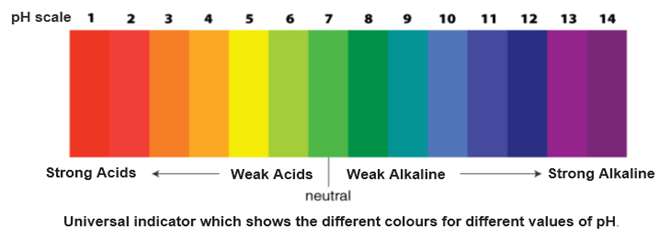
Universal Indicator
Q 1 – What happens when a blue litmus paper is dipped into an acid solution?
(a)Litmus paper turns red
(b) Litmus paper turns green
(c) Colour of litmus paper vanishes out
(d) No change in the color of litmus paper
(a) Litmus paper turns red
Q 2 – Assertion: Universal indicator gives green color with distilled water.
Reason: pH of distilled water is 7 and it is neutral and the universal indicator gives green color with a neutral solution.
(a) Both A and R are true and R is the correct explanation of A.
(b) Both A and R are true but R is not the correct explanation of A.
(c) A is true but R is false.
(d) A is false but R is true.
(e) Both A and R are false.
(a) Both A and R are true and R is the correct explanation of A.
Q 3 – What happens when a dry blue litmus paper is touched to acid powder?
(a) Litmus paper turns red
(b) Litmus paper turns green
(c) Colour of litmus paper vanishes out
(d) No change in the colour of litmus paper
(d) No change in the colour of litmus paper
Q 4 – The color of neutral litmus solution is
(a) red
(b) blue
(c) purple
(d) yellow
(c) purple
Q 5 – Which one is a suitable method to find the accurate pH value?
(a) pH meter
(b) pH paper
(c) Universal indicator
(d) Litmus solution
(a) pH meter
Q 6 – Which one of the following statements is correct about universal indicators?
(a) It is a mixture of HCl and NaOH
(b) It is a mixture of many indicators
(c) It is a solution of phenolphthalein in alcohol
(d) It is a solution of phenolphthalein in water.
(b) It is a mixture of many indicators
Q 7 – NaHCO, formed by reaction of
(a) NaOH + H2CO3
(b) NaCl + H2CO3
(c) Na2CO3 + HCl
(d) NaOH + Na2CO3
(a) NaOH + H2CO3
Q 8 – Bases dissociate which ions in their aqueous solution?
a. Hydroxide ions
b. Hydrogen ions
c. Chloride ions
d. None of the above
(a) Hydroxide ions
Q 9 – Which of the following properties are shown by dilute HCl?
(1) It turns blue litmus red
(2) It turns red litmus blue
(3) It reacts with zinc and gas is evolved
(4) It reacts with solid sodium carbonate to give brisk effervescence
(a) 1 and 2
(b) 1 and 3
(c) 1, 3 and 4
(d) 2, 3 and 4
(c) 1, 3 and 4
Q 10 – Eggshell is made up of
(a) CaCO3
(b) CaO
(c) Ca(OH)2
(d) CaCl2
(a) CaCO3
Q 11 – A teacher gave two test tubes – one containing water and the other containing sodium hydroxide solution to two students. Then he asked them to identify the test tube containing sodium hydroxide solution. Which one of the following can be used for correctly identifying the test tube containing the solution of sodium hydroxide?
(a) Blue litmus
(b) Red litmus
(c) Sodium carbonate solution
(d) Dilute HCl
(b) Red litmus
Q 12 – Which acid is found in tamarind?
a. Citric acid
b. Lactic acid
c. Tartaric acid
d. Sulphuric acid
(c) Tartaric acid
Q 13 – Which one of the following types of medicines is used for treating indigestion?
(a) Antibiotics
(b) Analgesic
(c) Antacid
(d) Antiseptic
(c) Antacid
Q 14 – Metallic oxides are ________ in nature, but non-metallic oxides are __________ in nature. The information in which alternative completes the given statement?
(a) neutral, acidic
(b) acidic, basic
(c) basic, neutral
(d) basic, acidic
(d) basic, acidic
Q 15 – Which acid is found in bee stings?
a. Citric acid
b. Formic acid
c. Tartaric acid
d. Nitric acid
(b) Formic acid
Q 16 – Two drops each of lemon juice, hydrochloric acid, and acetic acid were added separately to the blue litmus solution. Which of the following samples will change the color of the litmus?
(a) Lemon juice
(b) Acetic acid
(c) HCl
(d) Each one
(d) Each one.
Q 17 – Rain is called acid rain when it:
(a) pH falls below 7
(b) pH falls below 6
(c) pH falls below 5.6
(d) pH is above 7
(c) pH falls below 5.6
Q 18 – Which gas turns lime water milky?
(a) H2
(b) N2
(c) CO2
(d) SO2
(c) CO2
Q 19 – What happens when acid is mixed with water?
a. Heat is evolved
b. Heat is absorbed
c. Concentration of acid increases
d. All of the above
(a) Heat is evolved.
Q 20 – What is the pH range of the human body?
(a) 7.0 – 7.8
(b) 7.2 – 8.0
(c) 7.0 – 8.4
(d) 7.2 – 8.4
(a) 7.0 – 7.8
Q 21 – Lime water reacts with chlorine to give
(a) bleaching powder
(b) baking powder
(c) baking soda
(d) washing soda
(c) baking soda
Q 22 – What happens when an acid reacts with the base?
a. Acid neutralizes base
b. Water is formed
c. A salt is formed
d. All of the above
(d) All of the above
Q 23 – The water of crystallization of gypsum is
(a) 4
(b) 3
(c) 2
(d) 1
(c) 2
Q 24 – The pH values of distilled water, lemon juice, sodium bicarbonate were measured using pH paper. What is the correct decreasing order of pH values?
(a) water > lemon juice > sodium carbonate
(b) sodium carbonate > water > fruit juice
(c) lemon juice > water > sodium carbonate
(d) water > sodium carbonate > fruit juice
(b) sodium carbonate > water > fruit juice
Q 25 – Which of the following is an olfactory indicator?
a. Turmeric
b. Onion
c. Litmus
d. All of the above
(b) Onion
Q 26 – When a drop of unknown solution X is placed on a strip of pH paper, deep red color is produced. This sample is which one of these?
(a) NaOH
(b) HCl
(c) Water
(d) CH3COOH
(b) HCl
Q 27 – At what temperature is gypsum heated to form Plaster of Paris?
(a) 90°C
(b) 100°C
(c) 110°C
(d) 120°C
(c) 110°C
Q 28 – What happens when an acid is added to vanilla?
a. Color of vanilla changes into red
b. Vanilla becomes colorless
c. Vanilla loses its smell
d. Nothing happens
(d) Nothing happens
Q 29 – Which acid is produced in our stomach?
(a) HCl
(b) H2SO4
(c) Nitric acid
(d) Acetic acid
(a) HCl
Q 30 – Which formula represents bleaching powder?
(a) Ca(OH)2
(b) CaOCl2
(c) CaCl2
(d) CaO
(b) CaOCl2
Q 31 – Which of the following compounds is used in soda-acid fire extinguishers?
(a) calcium hydroxide
(b) calcium carbonate
(c) sodium hydrogen sulphate
(d) sodium hydrogen carbonate
(d) sodium hydrogen carbonate
Q 32 – N2CO3 . 10H2O is
(a) washing soda
(b) baking soda
(c) bleaching powder
(d) tartaric acid
(a) washing soda
Q 33 – Methyl orange which is an indicator turns into which color with an acid?
a. Red
b. Yellow
c. Pink
d. No color
(a) Red
Q 34 – Which one of the following is the correct method of finding the pH of a solution?
(a) heat the solution in a test tube and expose the pH paper to the vapors formed.
(b) pour few drops of solution from the test tube on the pH paper
(c) drop the pH paper in the solution
(d) put a drop of solution on the pH paper using a dropper
(d) put a drop of solution on the pH paper using a dropper
Q 35 – Which of the following compound is formed when zinc reacts with hydrochloric acid?
a. Zinc sulphate
b. Zinc chloride
c. Zinc carbonate
d. Zinc hydroxide
(b) Zinc chloride
Q 36 – The pH of which of the following samples can not be found directly using pH paper?
(a) Lemon juice
(b) Dilute HCl
(c) Solid sodium bicarbonate
(d) Solution of a detergent.
(c) Solid sodium bicarbonate
Q 37 – Tomato is a natural source of which acid?
(a) Acetic acid
(b) Citric acid
(c) Tartaric acid
(d) Oxalic acid
(d) Oxalic acid
Q 38 – Which of the following natural sources contains oxalic acid?
(a) lemon
(b) orange
(c) tomato
(d) tamarind
(c) tomato
Q 39 – Which of the following compound is formed when zinc reacts with sodium hydroxide?
a. Zinc hydroxide
b. Sodium zincate
c. Zinc hydrogenate
d. No reaction takes place
(b) Sodium zincate
Q 40 – The acid found in an ant sting is
(a) acetic acid
(b) citric acid
(c) tartaric acid
(d) methanoic acid
(d) methanoic acid
Q 41 – When hydrogen chloride gas is prepared on a humid day, the gas is usually passed through the guard tube containing calcium chloride. The role of calcium chloride taken in the guard tube is to
(a) absorb the evolved gas
(b) moisten the gas
(c) absorb moisture from the gas
(d) absorb Cl– ions from the evolved gas
(c) absorb moisture from the gas
Q 42 – If the pH value of a solution is 6, then solution will be of what type?
a. Strong acid
b. Strong base
c. Mild acid
d. Mild base
(c) Mild acid
Q 43 – To relieve pain caused due to acidity, we can take
(a) sour milk
(b) lemon juice
(c) orange juice
(d) milk of magnesia
(d) milk of magnesia
Q 44 – What are the products obtained when potassium sulphate reacts with barium iodide in an aqueous medium?
(a) KI and BaSO4
(b) KI, Ba and SO2
(c) K, I2 and BaSO4
(d) K, Ba, I2 and SO2
(a) KI and BaSO4
Q 45 – Assertion: Bleaching power liberate chlorine when kept in atmosphere.
Reason: CaOCl2 reacts with CO2 present in atmosphere to form CaCO3 and chlorine gas.
(a) Both A and R are true and R is the correct explanation of A.
(b) Both A and R are true but R is not the correct explanation of A.
(c) A is true but R is false.
(d) A is false but R is true.
(e) Both A and R are false.
(a) Both A and R are true and R is the correct explanation of A.
Q 46 – What happens when carbon dioxide gas reacts with sodium hydroxide?
a. Carbon monoxide is formed
b. Carbon dioxide is formed
c. Sodium carbonate is formed
d. Carbon dioxide does not react with sodium hydroxide
(c) Sodium carbonate is formed
Q 47 – Assertion: Ammonium hydroxide is Weak Base
Reason: Phenolphthalein becomes pink in NH4OH
(a) Both A and R are true and R is the correct explanation of A.
(b) Both A and R are true but R is not the correct explanation of A.
(c) A is true but R is false.
(d) A is false but R is true.
(b) Both A and R are true but R is not the correct explanation of A.
Q 48 – The concentration of H+ ions in a solution can be measured using
(a) a pH paper
(b) a litmus paper
(c) methyl orange
(d) phenolphthalein
(a) a pH paper
Q 49 – Which one of the following is required to identify the gas evolved when dilute hydrochloric acid reacts with zinc metal?
(a) blue litmus paper
(b) red litmus paper
(c) a burning slinter
(d) lime water
(c) a burning slinter
Q 50 – Which of the following acid does produce hydrogen ions in absence of water?
a. Hydrochloric acid
b. Sulphuric acid
c. Muriatic acid
d. None
(d) None
Q 51 – Zinc reacts with acid as well as with a base to liberate hydrogen. On the basis of this what should be the nature of the zinc metal?
(a) basic
(b) acidic
(c) amphoteric
(d) neutral
(c) amphoteric
Q 52 – Ag2S reacts with H2SO4 to form
(a) AgSO4
(b) Ag2SO4 + H2S
(c) Ag2O + H2S
(d) AgOH + H2S
(b) Ag2SO4 + H2S
We, at cbseinsights.com, have come up with the latest Acids-Bases and Salts For Class-10 Chemistry-MCQ as per the latest CBSE notification for 2021-22.

Q 1 – What happens when a blue litmus paper is dipped into an acid solution?
(a)Litmus paper turns red
(b) Litmus paper turns green
(c) Colour of litmus paper vanishes out
(d) No change in the color of litmus paper
(a)Litmus paper turns red
Explanation: An acid solution turns blue litmus paper red.
Q 2 – Assertion: Universal indicator gives green color with distilled water.
Reason: pH of distilled water is 7 and it is neutral and the universal indicator gives green color with a neutral solution.
(a) Both A and R are true and R is the correct explanation of A.
(b) Both A and R are true but R is not the correct explanation of A.
(c) A is true but R is false.
(d) A is false but R is true.
(e) Both A and R are false.
(a) Both A and R are true and R is the correct explanation of A.
Q 3 – What happens when a dry blue litmus paper is touched to acid powder?
(a) Litmus paper turns red
(b) Litmus paper turns green
(c) Colour of litmus paper vanishes out
(d) No change in the colour of litmus paper
(d) No change in the colour of litmus paper
Explanation: Acid powder does not change the colour of litmus paper. If blue litmus paper is dipped in the aqueous solution of acid then only blue litmus paper turns red. Water is necessary to change the colour of litmus paper because acid dissociates hydrogen ions in an aqueous solution.
Q 4 – The color of neutral litmus solution is
(a) red
(b) blue
(c) purple
(d) yellow
(c) purple
Q 5 – Which one is a suitable method to find the accurate pH value?
(a) pH meter
(b) pH paper
(c) Universal indicator
(d) Litmus solution
Ans. (a) pH meter
Q 6 – Which one of the following statements is correct about universal indicators?
(a) It is a mixture of HCl and NaOH
(b) It is a mixture of many indicators
(c) It is a solution of phenolphthalein in alcohol
(d) It is a solution of phenolphthalein in water.
Ans. (b) It is a mixture of many indicators
Q 7 – NaHCO, formed by reaction of
(a) NaOH + H2CO3
(b) NaCl + H2CO3
(c) Na2CO3 + HCl
(d) NaOH + Na2CO3
Ans. (a) NaOH + H2CO3
Q 8 – Bases dissociate which ions in their aqueous solution?
a. Hydroxide ions
b. Hydrogen ions
c. Chloride ions
d. None of the above
Ans. (a) Hydroxide ions
Q 9 – Which of the following properties are shown by dilute HCl?
(1) It turns blue litmus red
(2) It turns red litmus blue
(3) It reacts with zinc and gas is evolved
(4) It reacts with solid sodium carbonate to give brisk effervescence
(a) 1 and 2
(b) 1 and 3
(c) 1, 3 and 4
(d) 2, 3 and 4
Ans. (c) 1, 3 and 4
Q 10 – Eggshell is made up of
(a) CaCO3
(b) CaO
(c) Ca(OH)2
(d) CaCl2
Ans. (a) CaCO3
Q 11 – A teacher gave two test tubes – one containing water and the other containing sodium hydroxide solution to two students. Then he asked them to identify the test tube containing sodium hydroxide solution. Which one of the following can be used for correctly identifying the test tube containing the solution of sodium hydroxide?
(a) Blue litmus
(b) Red litmus
(c) Sodium carbonate solution
(d) Dilute HCl
Ans. (b) Red litmus
Q 12 – Which acid is found in tamarind?
a. Citric acid
b. Lactic acid
c. Tartaric acid
d. Sulphuric acid
Ans. (c) Tartaric acid
Q 13 – Which one of the following types of medicines is used for treating indigestion?
(a) Antibiotics
(b) Analgesic
(c) Antacid
(d) Antiseptic
Ans. (c) Antacid
Q 14 – Metallic oxides are ________ in nature, but non-metallic oxides are __________ in nature. The information in which alternative completes the given statement?
(a) neutral, acidic
(b) acidic, basic
(c) basic, neutral
(d) basic, acidic
Ans. (d) basic, acidic
Q 15 – Which acid is found in bee stings?
a. Citric acid
b. Formic acid
c. Tartaric acid
d. Nitric acid
Ans. (b) Formic acid
Q 16 – Two drops each of lemon juice, hydrochloric acid, and acetic acid were added separately to the blue litmus solution. Which of the following samples will change the color of the litmus?
(a) Lemon juice
(b) Acetic acid
(c) HCl
(d) Each one
Ans. (d) Each one
Q 17 – Rain is called acid rain when it:
(a) pH falls below 7
(b) pH falls below 6
(c) pH falls below 5.6
(d) pH is above 7
Ans. (c) pH falls below 5.6
Q 18 – Which gas turns lime water milky?
(a) H2
(b) N2
(c) CO2
(d) SO2
Ans. (c) CO2
Q 19 – What happens when acid is mixed with water?
a. Heat is evolved
b. Heat is absorbed
c. Concentration of acid increases
d. All of the above
Ans. (a) Heat is evolved
Q 20 – What is the pH range of the human body?
(a) 7.0 – 7.8
(b) 7.2 – 8.0
(c) 7.0 – 8.4
(d) 7.2 – 8.4
Ans. (a) 7.0 – 7.8
Q 21 – Lime water reacts with chlorine to give
(a) bleaching powder
(b) baking powder
(c) baking soda
(d) washing soda
Ans. (c) baking soda
Q 22 – What happens when an acid reacts with the base?
a. Acid neutralizes base
b. Water is formed
c. A salt is formed
d. All of the above
Ans. (d) All of the above
Q 23 – The water of crystallization of gypsum is
(a) 4
(b) 3
(c) 2
(d) 1
Ans. (c) 2
Q 24 – The pH values of distilled water, lemon juice, sodium bicarbonate were measured using pH paper. What is the correct decreasing order of pH values?
(a) water > lemon juice > sodium carbonate
(b) sodium carbonate > water > fruit juice
(c) lemon juice > water > sodium carbonate
(d) water > sodium carbonate > fruit juice
Ans. (b) sodium carbonate > water > fruit juice
Q 25 – Which of the following is an olfactory indicator?
a. Turmeric
b. Onion
c. Litmus
d. All of the above
Ans. (b) Onion
Q 26 – When a drop of unknown solution X is placed on a strip of pH paper, deep red color is produced. This sample is which one of these?
(a) NaOH
(b) HCl
(c) Water
(d) CH3COOH
Ans. (b) HCl
Q 27 – At what temperature is gypsum heated to form Plaster of Paris?
(a) 90°C
(b) 100°C
(c) 110°C
(d) 120°C
Ans. (c) 110°C
Q 28 – What happens when an acid is added to vanilla?
a. Color of vanilla changes into red
b. Vanilla becomes colorless
c. Vanilla loses its smell
d. Nothing happens
Ans. (d) Nothing happens
Q 29 – Which acid is produced in our stomach?
(a) HCl
(b) H2SO4
(c) Nitric acid
(d) Acetic acid
Ans. (a) HCl
Q 30 – Which formula represents bleaching powder?
(a) Ca(OH)2
(b) CaOCl2
(c) CaCl2
(d) CaO
Ans. (b) CaOCl2
Q 31 – Which of the following compounds is used in soda-acid fire extinguishers?
(a) calcium hydroxide
(b) calcium carbonate
(c) sodium hydrogen sulphate
(d) sodium hydrogen carbonate
Ans. (d) sodium hydrogen carbonate
Q 32 – N2CO3 . 10H2O is
(a) washing soda
(b) baking soda
(c) bleaching powder
(d) tartaric acid
Ans. (a) washing soda
Q 33 – Methyl orange which is an indicator turns into which color with an acid?
a. Red
b. Yellow
c. Pink
d. No color
Ans. (a) Red
Q 34 – Which one of the following is the correct method of finding the pH of a solution?
(a) heat the solution in a test tube and expose the pH paper to the vapors formed.
(b) pour few drops of solution from the test tube on the pH paper
(c) drop the pH paper in the solution
(d) put a drop of solution on the pH paper using a dropper
Ans. (d) put a drop of solution on the pH paper using a dropper
Q 35 – Which of the following compound is formed when zinc reacts with hydrochloric acid?
a. Zinc sulphate
b. Zinc chloride
c. Zinc carbonate
d. Zinc hydroxide
Ans. (b) Zinc chloride
Q 36 – The pH of which of the following samples can not be found directly using pH paper?
(a) Lemon juice
(b) Dilute HCl
(c) Solid sodium bicarbonate
(d) Solution of a detergent.
Ans. (c) Solid sodium bicarbonate
Q 37 – Tomato is a natural source of which acid?
(a) Acetic acid
(b) Citric acid
(c) Tartaric acid
(d) Oxalic acid
Ans. (d) Oxalic acid
Q 38 – Which of the following natural sources contains oxalic acid?
(a) lemon
(b) orange
(c) tomato
(d) tamarind
Ans. (c) tomato
Q 39 – Which of the following compound is formed when zinc reacts with sodium hydroxide?
a. Zinc hydroxide
b. Sodium zincate
c. Zinc hydrogenate
d. No reaction takes place
Ans. (b) Sodium zincate
Q 40 – The acid found in an ant sting is
(a) acetic acid
(b) citric acid
(c) tartaric acid
(d) methanoic acid
Ans. (d) methanoic acid
Q 41 – When hydrogen chloride gas is prepared on a humid day, the gas is usually passed through the guard tube containing calcium chloride. The role of calcium chloride taken in the guard tube is to
(a) absorb the evolved gas
(b) moisten the gas
(c) absorb moisture from the gas
(d) absorb Cl– ions from the evolved gas
Ans. (c) absorb moisture from the gas
Q 42 – If the pH value of a solution is 6, then solution will be of what type?
a. Strong acid
b. Strong base
c. Mild acid
d. Mild base
Ans. (c) Mild acid
Q 43 – To relieve pain caused due to acidity, we can take
(a) sour milk
(b) lemon juice
(c) orange juice
(d) milk of magnesia
Ans. (d) milk of magnesia
Q 44 – What are the products obtained when potassium sulphate reacts with barium iodide in an aqueous medium?
(a) KI and BaSO4
(b) KI, Ba and SO2
(c) K, I2 and BaSO4
(d) K, Ba, I2 and SO2
Ans. (a) KI and BaSO4
Q 45 – Assertion: Bleaching power liberate chlorine when kept in atmosphere.
Reason: CaOCl2 reacts with CO2 present in atmosphere to form CaCO3 and chlorine gas.
(a) Both A and R are true and R is the correct explanation of A.
(b) Both A and R are true but R is not the correct explanation of A.
(c) A is true but R is false.
(d) A is false but R is true.
(e) Both A and R are false.
Ans. (a) Both A and R are true and R is the correct explanation of A.
Q 46 – What happens when carbon dioxide gas reacts with sodium hydroxide?
a. Carbon monoxide is formed
b. Carbon dioxide is formed
c. Sodium carbonate is formed
d. Carbon dioxide does not react with sodium hydroxide
Ans. (c) Sodium carbonate is formed
Q 47 – Assertion: Ammonium hydroxide is Weak Base
Reason: Phenolphthalein becomes pink in NH4OH
(a) Both A and R are true and R is the correct explanation of A.
(b) Both A and R are true but R is not the correct explanation of A.
(c) A is true but R is false.
(d) A is false but R is true.
Ans. (b) Both A and R are true but R is not the correct explanation of A.
Q 48 – The concentration of H+ ions in a solution can be measured using
(a) a pH paper
(b) a litmus paper
(c) methyl orange
(d) phenolphthalein
Ans. (a) a pH paper
Q 49 – Which one of the following is required to identify the gas evolved when dilute hydrochloric acid reacts with zinc metal?
(a) blue litmus paper
(b) red litmus paper
(c) a burning slinter
(d) lime water
Ans. (c) a burning slinter
Q 50 – Which of the following acid does produce hydrogen ions in absence of water?
a. Hydrochloric acid
b. Sulphuric acid
c. Muriatic acid
d. None
Ans. (d) None
Q 51 – Zinc reacts with acid as well as with a base to liberate hydrogen. On the basis of this what should be the nature of the zinc metal?
(a) basic
(b) acidic
(c) amphoteric
(d) neutral
Ans. (c) amphoteric
Q 52 – Ag2S reacts with H2SO4 to form
(a) AgSO4
(b) Ag2SO4 + H2S
(c) Ag2O + H2S
(d) AgOH + H2S
Ans. (b) Ag2SO4 + H2S
Q 53 – When you test the solutions of sodium bicarbonate, sodium hydroxide, hydrochloric acid, and acetic acid with a universal indicator, in which case would you get a red color?
(a) sodium bicarbonate
(b) hydrochloric acid
(c) sodium hydroxide
(d) acetic acid
Ans. (b) hydrochloric acid
Q 54 – What is the color of anhydrous copper sulphate?
a. Blue
b. White grey
c. Blue-grey
d. Greenish
Ans. (b) White grey
Q 55 – A sample of soil is mixed with water and allowed to settle. The clear supernatant solution turns the pH paper yellowish-orange.
Which of the following would change the color of this pH paper to greenish-blue?
(a) Lemon juice
(b) Vinegar
(c) Common salt
(d) An antacid
Ans. (d) An antacid
Q 56 – Which of the following is known as soda ash?
a. Sodium carbonate
b. Sodium bicarbonate
c. Sodium carbonate decahydrate
d. Calcium carbonate
Ans. (a) Sodium carbonate
Q 57 – The pH of a sample of pure water is 7 at room temperature. What is its pH when a pinch of solid sodium bicarbonate is dissolved in it?
(a) vary near to 7
(b) less than 7
(c) more than 7
(d) exactly 7
Ans. (c) more than 7
Q 58 – What is the chemical name of baking powder?
a. Sodium carbonate
b. Sodium bicarbonate
c. Potassium carbonate
d. Calcium carbonate
Ans. (b) Sodium bicarbonate
Q 59 – How many water molecules does hydrated calcium sulphate contain?
(a) 5
(b) 10
(c) 7
(d) 2
Ans. (d) 2
Q 60 – When copper oxide and dilute hydrochloric acid react, colour changes to
(a) white
(b) bluish-green
(c) blue-black
(d) black
Ans. (b) bluish-green
Q 61 – Baking soda is produced by which of the following process?
a. Chlor-alkali process
b. Solvay process
c. Soda process
d. Dobereiner’s Triads process
Ans. (b) Solvay process
Q 62 – Nettle sting is a natural source of which acid?
(a) Methanoic acid
(b) Lactic acid
(c) Citric acid
(d) Tartaric acid
Ans. (a) Methanoic acid (HCOOH)
Q 63 – The H+ ion concentration of a solution is 1.0 x 10–5 m. The solution is
(a) Acidic
(b) Alkaline
(c) Neutral
(d) Amphoteric
Ans. (a) Acidic
Q 64 – In terms of acidic strength, which one of the following is in the correct increasing order?
(a) Water < Acetic acid < Hydrochloric acid
(b) Water < Hydrochloric acid < Acetic acid
(c) Acetic acid < Water < Hydrochloric acid
(d) Hydrochloric acid < Water < Acetic acid
Ans. (a) Water < Acetic acid < Hydrochloric acid
Q 65 – Setting of Plaster of Paris takes place due to
(a) Oxidation
(b) Reduction
(c) Dehydration
(d) Hydration
Ans. (d) Hydration
Assertion and Reason Questions…….
The following questions consist of two statements – Assertion (A) and Reason (R). Answer these questions selecting the appropriate option given below:
(a) Both A and R are true and R is the correct explanation of A.
(b) Both A and R are true but R is not the correct explanation of A.
(c) A is true but R is false.
(d) A is false but R is true.
Q 66 – Assertion (A): Copper sulphate crystals are wet because they contain water of crystallization.
Reason (R): Water of crystallization is the fixed number of molecules of water present in one formula unit of salt.
Ans. (d) A is false but R is true.
Q 67 – Assertion (A): Strong acids have low electrical conductivity.
Reason (R): Strong acids and weak acids have an equal concentration of hydrogen ions in their solutions.
Ans. (d) A is false but R is true.
Q 68 – Assertion (A): During electrolysis of a concentrated aqueous solution of sodium chloride, hydrogen is produced at the cathode and chlorine gas is produced at the anode.
Reason (R): Ions get attracted to oppositely charged electrodes.
Ans. (a) Both A and R are true and R is the correct explanation of A.
Q 69 –Assertion (A): The chemical formula of bleaching powder is CaoCl2.
Reason (R): Calcium oxide reacts with chlorine to form bleaching powder.
Ans. (c) A is true but R is false.
Q 70 –Assertion: The process of dissolving an acid or a base in water is a highly exothermic reaction.
Reason: Water must always be added slowly to acid with constant stirring.
Ans. (c) A is true but R is false.
Explanation:- Always acid is dissolved slowly to water with constant stirring.
Q 71 –Assertion: Hydrochloric Acid (HCl) is a stronger acid than Acetic acid (CH3COOH).
Reason: On dissociation, HCl yields lesser hydrogen ions for the same concentration as compared to acetic acid.
Ans. (c) A is true but R is false.
Q 72 – Assertion: pH of ammonium nitrate solution (NH4NO3) is acidic.
Reason: The ammonium nitrate solution is a salt of a weak base (NH4OH) and a strong acid (HNO3) which will always be acidic in nature.
Ans. (a) Both A and R are true and R is the correct explanation of A.
Q 73 – Assertion: Higher the H+ ion concentration, the lower its pH value.
Reason: The pH of a neutral solution = 7, that of a basic solution < 7, and that of an acidic solution > 7.
Ans. (c) A is true but R is false.
Q 74 – Assertion: CH3COOH is used like vinegar in cooking and food preservatives.
Reason: Strong acids are those acids that ionize almost completely in an aqueous solution and hence produce a large amount of H+ ions.
Ans. (b) Both A and R are true but R is not the correct explanation of A.
Q 75 – Assertion: Milk of Magnesia Mg(OH)2 neutralizes the effect of extra acid produced in the stomach during indigestion and thus provides relief.
Reason: Milk of Magnesia Mg(OH)2 is a mild base.
Ans. (a) Both A and R are true and R is the correct explanation of A.
Q 76 – Plaster of Paris hardens by
(a) Giving off CO2
(b) Changing into CaCO3
(c) Combining with water
(d) Giving out water
Ans. (c) Combining with water CaCO3
Q 77 – The odor of acetic acid resembles that of
(a) Rose
(b) Burning Plastic
(c) Vinegar
(d) Kerosene
Ans. (c) Vinegar
Q 78 – The H+ ion concentration of a solution is 1.0×10–5m. The solution is
(a) Acidic
(b) Alkaline
(c) Neutral
(d) Amphoteric
Ans. (a) Acidic
Q 79 – Chemical formula of baking soda is:
(a) MgSO4
(b) Na2CO3
(c) NaHCO3
(d) MgCO3
Ans. (c) NaHCO3
Q 80 – An aqueous solution turns red litmus solution blue. Excess addition of which of the following solution would reverse the change?
(a) Baking powder
(b) Lime
(c) Ammonium hydroxide solution
(d) Hydrochloric acid
Ans. (d) Hydrochloric acid