We, at cbseinisghts.com , have come up with Carbon and its Compound For Class-10 Chemistry-MCQ so as to increase the competitive skills of the students. Consequently, the students will be able to have a quick view of the major concepts related to the chapter.
Q 1 – Magnesium ribbon is cleaned before burning. Why?
A. It absorbs moisture from the atmosphere which interferes with burning.
B. It’s outer layer form oxides which interfere with burning.
C. Dust from the atmosphere prevents its burning.
D. All of the above
B. It’s outer layer form oxides which interfere with burning.
Q 2 – The correct electron dot structure of a water molecule is

(C)
Q 3 – What is the formula for quick lime. What does it form when put into water.
A. CaO, CaCO3
B. CaO, Ca(OH)2
C. Ca(OH)2 , CaCO3
D. None
B. CaO, Ca(OH)2
Q 4 – Electrolysis of water is
A. Combination reaction
B. Decomposition reaction
C. Displacement reaction
D. None
B. Decomposition reaction
Q 5 – A hydrocarbon reacts with ammonical cuprous chloride solution to form a red precipitate, the hydrocarbon is –
(a) Ethane
(b) ethane
(c) butane
(d) 1-propyne
(d) 1-propyne
Q 6 – Example of displacement reaction is
(a) Fe(s) + CuSO4 (aq) ——> FeSO4 (aq) + Cu(s)
(b) C(s) + O2 (g) ——–> CO2 (g)
Sunlight
(c) AgCl(s) ———> 2Ag(s) + Cl2 (g)
(d) None
(a) Fe(s) + CuSO4 (aq) ——> FeSO4 (aq) + Cu(s)
Q 7 – Which of the following is not an allotropic form of carbon ?
(a) fluorine
(b) fullerene
(c) diamond
(d) graphite
(a) fluorine
Q 8 – Graphite is used as a lubricant in machines because
(a) it is a good conductor of electricity.
(b) it has a high melting point and slippery layers.
(c) its density ranges from 1.9 to 2.3 g/cm3 .
(d) it is strong and soft
(b) it has a high melting point and slippery layers.
Q 9 – Lead nitrate Pb(NO3)2 on heating forms Lead oxide (PbO) solid and Nitrogen dioxide gas. What are the color of lead oxide and nitrogen dioxide?
A. White, Colourless
B. White, Brown
C. Yellow, Brown
D. Yellow, Colourless
C. Yellow, Brown
Q 10 – Identify the compound that undergoes bromination reaction:

(d)
Q 11 – Quick lime is used in whitewashing because
A. It is cheap
B. It forms slaked lime with water which has a nice color.
C. It forms slaked lime with water which reacts with atmospheric carbon dioxide to form limestone.
D. None
C. It forms slaked lime with water which reacts with atmospheric carbon dioxide to form limestone.
Q 12 – Which of the following represents the correct deceasing order of hydrogen atoms ?
(a) alkanes, alkenes, alkynes
(b) alkanes, alkynes, alkenes
(c) alkenes, alkynes, alkanes
(d) alkynes, alkanes, alkenes
(a) alkanes, alkenes, alkynes
Q 13 – Example of a double displacement reaction will be
A. Metal with a salt
B. Metal with an acid
C. Metal with metal
D. Salt with a salt
D. Salt with a salt
Q 14 – The allotrope of carbon which is a good conductor of heat and electricity is
(a) diamond
(b) graphite
(c) charcoal
(d) none of these
(b) graphite
Q 15 – Ferrous sulfate solution is green while ferric oxide formed by its decomposition is
A. Red
B. White
C. Brown
D. Yellow
C. Brown
Q 16 – Identify the functional group present in the following compound
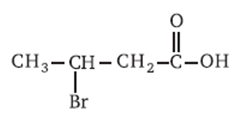
(a) aldehyde
(b) bromine
(c) carboxylic
(d) both bromine and carboxylic group
(d) both bromine and carboxylic group
Q 17 – Rancidity of fat or oil is:
A. Degradation by the microorganism
B. Oxidation of fat
C. Reduction of fat
D. None
B. Oxidation of fat
Q 18 – In double covalent bond there is a sharing of
(a) 2 electrons
(b) 4 electrons
(c) 6 electrons
(d) 3 electrons
(b) 4 electrons
Q 19 – The oxidation reaction is:
A. Addition of an oxygen atom
B. Removal of the hydrogen atom
C. Loss of electron
D. All
D. All
Q 20 – Lead pencil contains
(a) graphite
(b) diamond
(c) lead
(d) lead sulphate
(a) graphite
Q 21 – Name the following aromatic compound
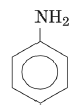
(a) toluene
(b) aniline
(c) phenol
(d) furan
(a) toluene
Q 22 – The blue flame in the below image represents the burning of which metal?

A. Iron
B. Copper
C. Sodium
D. All metal burn to give a blue flame.
B. Copper
Q 23 – Which of the following is not a straight chain?
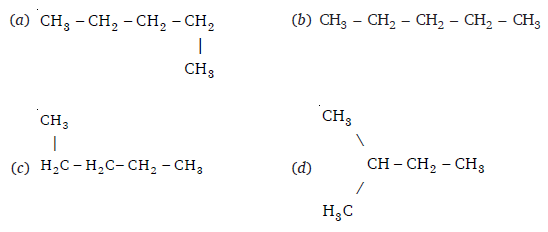
(d)
Q 24 – Mineral acids are stronger acids than carboxylic acids because
(i) mineral acids are completely ionised
(ii) carboxylic acids are completely ionised
(iii) mineral acids are partially ionized
(iv) carboxylic acids are partially ionised
(a) (i) and (iv)
(b) (ii) and (iii)
(c) (i) and (ii)
(d) (iii) and (iv)
(d) butane and 2-methyl propane
Ans –
Ans –
Q 25 – Cation is formed when
(a) atom gains electrons
(b) atom losses electrons
(c) proton is lost by the atom
(d) atom shared by electrons
Ans- (b) atom losses electrons
Q 26 – The structural formula of ethyl ethanoate
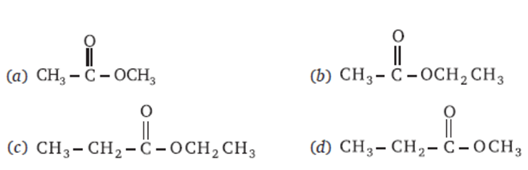
Ans – (b)
Q 27 – When ethyl alcohol and acetic acid are mixed, the resulting ester has a chemical formula
(a) CH3COOC2H5
(b) C2H5COOCH3
(c) C2H5COOC2H5
(d) CH3COOCH3
Ans – (a) CH3COOC2H5
Q 28 – The compound which gives a brisk effervescence with sodium metal and not with sodium hydrogen carbonate is
(a) ethanol
(b) ethanoic acid
(c) both ethanoic acid and ethanol
(d) none of these
Ans – (a) ethanol
Q 29 – Which of the following has the weakest carbon-carbon strength?
(a) C2H2
(b) C2H4
(c) C2H6
(d) all have the same bond strength
Ans – (a) C2H2
Q 30 – Which is denatured spirit?
(a) ethanol only
(b) ethanol and methanol (50%)
(c) ethanol and methanol (5%)
(d) methanol only
Ans – (c) ethanol and methanol (5%)
Q 31 – Acetic acid was added to a solid X kept in a test tube. A colourless, odourless gas Y was evolved. The gas was passed through lime water, which turned milky. It was concluded that
(a) solid X is sodium hydroxide and the gas Y is CO2
(b) solid X is sodium bicarbonate and the gas Y is CO2
(c) solid X is sodium acetate and the gas Y is CO2
(d) solid X is sodium bicarbonate and the gas Y is SO2
Ans – (b) solid X is sodium bicarbonate and the gas Y is CO2
Q 32 – An organic compound ‘X’ has the molecular formula C3H6O2. It has a pleasant smell but does not turn blue litmus red. It has structural formula
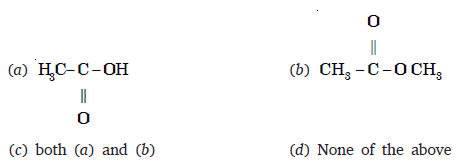
Ans – (b)
Q 33 – Drinking alcohol and driving may cause serious accidents. To discourage this, police randomly test drivers for alcohol using a breath analyser. The breath analyser works because
(a) Alcohol makes the breath dry and the machine registers moisture
(b) Alcohol makes the breath hotter which changes the machine reading
(c) Alcohol causes more saliva which the machine checks.
(d) Alcohol in the breath cause a chemical change registered by the machine
Ans – (b) Alcohol makes the breath hotter which changes the machine reading
Q 34 – Which of the following is not a saturated hydrocarbon ?
(a) cyclohexane
(b) benzene
(c) butane
(d) isobutane
Ans – (b) benzene
Q 35 – Tertiary butane gets oxidised with oxidizing agents like alkaline KMNO4 to
(a) Isobutane
(b) Ter-butyl alcohol
(c) Secondary-propyl alcohol
(d) All of above
Ans – (b) Ter-butyl alcohol
Q 36 – Which of the following salt when dissolved in water produce hard water ?
(a) calcium sulphate
(b) magnesium bicarbonate
(c) calcium chloride
(d) any of the above.
Ans – (d) any of the above.
Q 37 –

In the above given reaction, alkaline KMnO acts as
(a) reducing agent
(b) oxidising agent
(c) catalyst
(d) dehydrating agent
Ans – (b) oxidising agent
Q 38 – The by product of soap is
(a) isoprene
(b) glycerol
(c) butene
(d) ethylene glycol
Ans – (b) glycerol
Q 39 – The functional group present in a carboxylic acid is

Ans – (b)
Q 40 – Covalent compounds
(a) have high melting and boiling points
(b) are mostly soluble in water
(c) are formed between atoms of metals and non-metals
(d) are formed by the sharing of electrons in the bonding atoms.
Ans- (d) are formed by the sharing of electrons in the bonding atoms.
Q 41 – Which of the following statements are usually correct for carbon compounds? These
(i) are good conductors of electricity
(ii) are poor conductors of electricity
(iii) have strong forces of attraction between their molecules
(iv) do not have strong forces of attraction between their molecules
(a) (i) and (iii)
(b) (ii) and (iii)
(c) (i) and (iv)
(d) (ii) and (iv)
Ans – (d) (ii) and (iv)
Q 42 – The heteroatoms present is CH3 – O – CH2 – CH2 (Br)
(a) oxygen
(b) carbon
(c) hydrogen
(d) bromine
Ans – (d) bromine
Q 43 – Vinegar is a solution of
(a) 30% – 40% acetic acid in alcohol
(b) 5% – 8% acetic acid in alcohol
(c) 5% – 8% acetic acid in water
(d) 15% – 20% acetic acid in water
Ans – (c) 5% – 8% acetic acid in water
Q 44 – Acetic acid was added to a liquid X kept in a test tube. A colourless and odourless gas Y was evolved. The gas was passed through lime water which turned milky. It was concluded that:
(a) Liquid X is sodium hydroxide and the gas Y is CO2
(b) Liquid X is sodium carbonate and the gas Y is CO2
(c) Liquid X is sodium acetates and the gas Y is CO2
(d) Liquid X is sodium chloride and the gas Y is SO2
Ans – (b) Liquid X is sodium carbonate and the gas Y is CO2
Q 45 – Ethanol acid is also known as which of these?
(a) Formic acid
(b) Ethyl alcohol
(c) Ethane
(d) Acetaldehyde
Ans – (b) Ethyl alcohol
Q 46 – The number of single and double bonds present in benzenes are
(a) 9 and 6
(b) 9 and 3
(c) 12 and 3
(d) 12 and 6
Ans – (b) 9 and 3
Q 47 – The upper and lower homologue of C2H5OH are respectively
(a) methyl alcohol and butyl alcohol
(b) ethyl alcohol and propyl alcohol
(c) butyl alcohol and propyl alcohol
(d) propyl alcohol and methyl alcohol
Ans – (d) propyl alcohol and methyl alcohol
Q 48 – The general formula for alkanes is CnH2n+1– CHO. The value of ‘n’ for the first member.
(a) 1
(b) 0
(c) 0.5
(d) 1.1
Ans- (b) 0