Q 1. What are good conductors?
Ans. The substances that conduct electricity through them are called good conductors.

Q 2. Most liquids which conduct electricity are solutions of _________ and __________.
Ans. salt, water
Q 3. What are insulators or poor conductors?
Ans. The substances that do not conduct electricity through them are poor conductors or insulators.
Q 4. Give four examples of conductors.
Ans. Copper, iron, aluminium and brass.
Q 5. Do some liquids also conduct electricity?
Ans. Yes, some liquids also conduct electricity, e.g., mercury (metal), impure water, an aqueous solution of acids, bases, etc.
Q 6. When current flows in a wire, a compass needle kept nearby gets defected. What effect of current is this?
Ans. This is the magnetic effect of current.
Q 7. Give four examples of insulators.
Ans. Air, wood, rubber and plastic.
Q 8. Name two metal objects which have a coating of another metal.
Ans. Handlebars of bicycles, bathroom taps.
Q 9. Can there be made a coating of one metal on another?
Ans. Yes, by electroplating, it can be done.
Q 10. What do we get on the electrolysis of acidified water?
Ans. Hydrogen and oxygen gas.
Q 11. Is air a bad or good conductor?
Ans. A bad conductor.
Q 12. Which metal is plated on handlebars of cycles and rim of wheels?
Ans. Chromium
Q 13. What is the full form of an LED?
Ans. Light Emitting Diode.
Q 14. In electroplating of copper, a layer of copper builds upon the plate. But, what about the loss of copper from the solution?
Ans. From the other electrode, an equal amount of copper gets dissolved in the solution.
Q 15. Why chromium is electroplated on other inferior materials?
Ans. Because it has a shiny appearance, it does not corrode and resists scratches as well.
Q 16. How do we check the electric current?
Ans. We check the electric current by using a tester.
Q 17. Which part of an atom is responsible for flow of current?
Ans. Electron

Q 18. Why do most liquids conduct electricity?
Ans. Due to the presence of ions in them, most liquids conduct electricity.
Q 19. An LED is a more efficient device than a bulb. Why?
Ans. LED is more efficient because it can glow even when a weak or less current flows through it.
Q 20. Do lemon juice or vinegar conduct electricity?
Ans. Yes, they conduct electricity.
Q 21. How is the conductivity of liquids tested?
Ans. : By using a tester.
Q 22. Is water from taps, handpumps, wells and ponds a good conductor?
Ans. Yes, water from these sources is a good conductor.
Q 23. What makes distilled water a good conductor?
Ans. Salts when mixed with distilled water make it a good conductor.
Q 24. Why is a layer of zinc-coated over iron?
Ans. To prevent iron from corrosion and rust.
Q 25. Why is tap water, a conductor of electricity?
Ans. Tap water contains lots of mineral salts dissolved in it which make this water conductor of electricity.
Q 26. Why is distilled water an insulator of electricity?
Ans. Distilled water is free from impurities like salts, minerals, dust, etc., so it is an insulator of electricity.
Q 27. Will the solution of sugar in distilled water conduct electricity?
Ans. No
Q 28. Why is tin electroplated on iron to make cans used for storing food?
Ans. Tin is less reactive than iron. The tin coating prevents food from coming in contact with iron and thus prevents it from getting spoiled or corroded.
Q 29. Why do we use chromium electroplating on taps and bars of bicycles instead of silver and gold?
Ans. Silver and gold are very expensive compared to chromium.
Q 30. What type of effect of current do the deposits of metal on electrodes show?
Ans. Chemical effect
Q 31. What effect of current does electroplating show?
Ans. Chemical effect
Q 32. Which effect of current causes the bulb to glow?
Ans. Heating effect
Q 33. Define insulators.
Ans. Materials, which do not allow an electric current to pass through them are known as insulators or non-conductor, e.g., rubber, plastic, wood, paper, etc.
Q 34. Which part of the bulb glows?
Ans. Filament
Q 35. What precaution is taken in regard to the cell, while checking the tester? Why?
Ans. While checking the tester, one must not join its free ends for more than a few seconds. Otherwise, the cells of the battery will drain very quickly.
Q 36. Name the three effects of electric current.
Ans. Heating, magnetic and chemical effect.
Q 37. How are tin cans made of? Why are tin cans used for storing food items?
Ans. Tin cans used for storing food items are made by electroplating a layer of the tin onto the iron. The tin is less reactive as compared to iron. Thus, the food is protected against spoilage.
Q 38. How can the magnetic effect of current be checked?
Ans. By using a magnetic compass.
Q 39. What do we see when the compass needle is brought near a wire in which current is flowing?
Ans. The needle deflects.
Q 40. Why is tap water, a conductor of electricity?
Ans. Tap water contains lots of mineral salts dissolved in it which make this water conductor of electricity.
Q 41. Why is distilled water an insulator of electricity?
Ans. Distilled water is free from impurities like salts, minerals, dust, etc., so it is an insulator of electricity.
Q 42. What is electroplating?
Ans. Deposition of a thin layer of metal over other metals by electrolysis is called electroplating.
Q 43. Define good conductors and poor conductors or insulators.
Ans. The materials that conduct electricity through them are called good conductors whereas those that do not conduct electricity are called poor conductors or insulators. For example, copper, brass, aluminium, iron, etc., are conductors whereas rubber, plastic, wood, air, etc., are insulators.
Q 44. In electroplating of copper, a layer of copper builds upon the plate. But, what about the loss of copper from the solution?
Ans. From the other electrode, an equal amount of copper gets dissolved in the solution.
Q 45. Why chromium is electroplated on other inferior materials?
Ans. Because it has a shiny appearance, it does not corrode and resists scratches as well.
Q 46. What happens when an electric current is passed through the conducting solution?
Ans. The passage of an electric current through a conducting solution causes chemical reactions. As a result, bubbles of gas may be formed, deposits of metal on electrodes may be seen and changes of colour of the solution may occur, depending on what solutions and electrodes are used. This is called the chemical effects of the electric current.
Q 47. How is the conductivity of liquids tested?
Ans. The free ends of the tester are dipped in the liquid. If the bulb glows, the liquid is said to be a conductor. If not, it is an insulator.
Q 48. Show with the help of a diagram that lemon juice and vinegar are good conductors of electricity.
Ans. When the ends of a tester are dipped in lemon juice or vinegar, the bulb glows. This process indicates that lemon juice and vinegar, both, are good conductors of electricity.

Q 49. What is an LED? Why is it preferred to another type of bulb?
Ans. The electric device which is used in the tester instead of the bulb is an LED. Its full form is Light Emitting Diode.
It is preferred to other bulbs as it can glow even when weak or less current sows through it.
Q 50. Explain the conductivity of water.
Or
Normal water conducts electricity while pure or distilled water does not. Explain why?
Ans. Normal water that we get from sources such as taps, handpumps, wells, ponds, etc., is not pure. It may contain several salts dissolved in it naturally. This water is thus a good conductor of electricity. Pure or distilled water is free of salts and is a poor conductor.
Q 51. Explain the working of a lightning conductor.
Ans. A lightning conductor is a safety device that protects a tall .building from lightning strikes, by providing an easy and alternative path to the heavy charge of lightning. It consists of a long metal rod fixed with the sidewall of a tall building such that its upper end
like Trishul much above the building while the lower end runs deep under the earth and joins a large metal plate. Whenever there is lightning, the heavy charge of the lightning is conducted through the metal rod to the earth and the building remains safe.
Q 52. Give an example of the chemical effect of the electric current.
Ans. The passage of an electric current through a conducting solution causes chemical reactions as a result, bubbles of gas are formed, deposits of metal are seen on electrodes or changes in the colour of the solution, may occur. These are some of the chemical effects of electric current.
Q 53. What is electroplating? What are its uses?
Ans.
The process of depositing a layer of any desired metal on another material by means of electricity is called electroplating.
Electroplating is a very useful process. This is used to make objects appear shiny and resistant to scratches. It prevents corrosion.
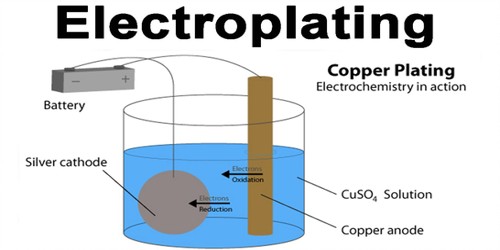
Q 54. What happens when an electric current is passed through the copper sulphate solution?
Ans. When an electric current is passed through the copper sulphate solution, copper sulphate dissociates into copper and sulphate. The free copper gets drawn to the electrode connected to the negative terminal of the battery and gets deposited on it.
Q 55. Why is the disposal of the used conducting solution in the electroplating factories a major concern?
Ans. In electroplating factories, the used conducting solution is a hazardous waste that creates a lot of pollution. That is why the disposal of the used conducting solution is a major concern.
Q 56. How does a bulb glow in liquid? Explain.
Ans. When the liquid between the two ends of a tester allows the electric current to pass through it, then the circuit of the tester becomes complete and the current flow in the circuit of liquid to make the bulb glow. But when the liquid does not allow the electric current to pass through it, then the circuit of the tester is incomplete and the bulb does not glow.
Q 57. What is the magnetic effect of electricity? Explain.
Ans. When an electric current is passed through a coil of wire, it behaves like a magnet. This is known as the magnetic effect of current. It depends on the amount of current passing through the coil of wire.
Q 58. Why is a magnetic compass needed to test the conduction of electric current?
Ans. Sometimes, when the current passing through a conductor is so small that filament of the bulb does not get heated up and the bulb does not glow. In this case, we need a magnetic compass to test the conduction of current.
Q 59. What happens when an electric current is passed through a cut potato for a considerable time?
Ans. When an electric current is passed through a cut potato for a considerable time, a greenish-blue spot is formed around the positive electrode. The chemical effect of the electric current is involved in this process.
Q 60. Why is chromium used for electroplating? Why do the objects have chromium plating that is not made of chromium itself?
Ans. Chromium has a shiny look. It does not get corroded and it resists scratches. Chromium is however expensive and it may not be economical to make the whole object out of it. So the object is made from a cheaper metal and only a coating of chromium is done over it.
Q 61. Which metals, except chromium, are used for electroplating other metals?
Ans. Jewellery makers electroplate silver and gold on ornaments of less expensive metals. Tin cans, used for storing food, are made by electroplating tin onto the iron. Tin is less reactive than iron. Hence, food is protected from getting spoilt. Iron used in bridges and automobiles is coated with zinc to protect them from corrosion and the formation of rust.
Q 62. Current does not flow in a circuit if there is a gap between the two wires. Does it indicate that air is a poor conductor of electricity? Does air never conduct electricity? Explain.
Ans. Air is a poor conductor of electricity if it is dry but in certain cases like during lightning and when air is moist, air may conduct electricity.
Q 63. On what factors thickness of the electroplated items depend?
Ans. The thickness of electroplated items depend upon:
- The strength of the current passing through the circuit.
- The concentration of the metal ion in the solution.
- The duration of the time the article has been in the solution.
Q 64. What is the benefit of chromium plating on other cheaper materials?
Ans. Chromium has a shiny appearance. It does not corrode and resists scratches. However, chromium is expensive and cannot be used to make the whole object. So, the object is made from a cheaper metal and only a coating of chromium over it is done.
Q 65. With the help of a suitable diagram, explain the electrolytic refining of copper.

Ans. To purify copper, a thin plate of pure copper and a thick rod of impure copper are used as electrodes in the acidified solution of CuS04. Pure copper is used as a cathode and impure copper is used as the anode. When an electric current is passed through the copper sulphate solution, copper sulphate dissociates into copper and sulphate. The free copper gets drawn to the electrode connected to the negative terminal of the battery and gets deposited on it. From the impure copper electrode, an equal amount of copper gets dissolved in the solution. Thus, the loss of copper from the solution is restored and the process continues. The impurities are left behind at the anode.
Q 66. Does water conduct electricity? Show with the help of an activity.
Or
Show the conductivity of water with the help of an activity.
Ans.
Normal or ordinary water is a good conductor of electricity while distilled water is a bad conductor or insulator. Ordinary water may contain a small number of mineral salts dissolved in it naturally; on the other hand, distilled water is free of salts.
The following activity shows this fact:
About 50 ml of distilled water is taken in a clean and dry beaker. When the tester is dipped into the distilled water, the bulb does not glow which shows that distilled water is a bad conductor of electricity. But when a small amount of common salt is dissolved in distilled water and again tested the bulb glows which shows that distilled water when mixed with salts conduct electricity.
Q 67. What is electroplating? On which effect of the electric current is it based? Why is it done?
Ans. The process of depositing or coating a layer of any desired metal on the surface of other material by means of electricity is called electroplating. It is one of the most common applications of the chemical effects of electric current.
Electroplating is a very useful process. It is widely used in industry for coating metal objects with a thin layer of a different metal. The layer of metal deposited has some desired property, which the metal of the object lacks. For example, chromium plating is done on many objects to make them shiny and attractive.
Q 68. What are the advantages and disadvantages of electroplating?
Ans. Electroplating is a very useful process. It is widely used in industry for coating metal objects with a thin layer of a different metals.
The advantages and disadvantages of electroplating are:
Advantages:
- It protects the metals from being corroded.
- It prevents the rusting of metals.
- It makes cheap and dull metals shiny and attractive.
- It can make more reactive metals like iron less reactive.
- Chromium coating on metals give lustre to objects.
Disadvantages
- Pollutants from electroplating industries are very harmful. Some chemicals are very lethal for both human and animals.
- It is an expensive process.
Q 69. Why electric fires are extinguished with either using CO extinguisher or mud but not water?
Ans. As water is a good conductor of electricity so it can cause electrocution. Hence, water is avoided in extinguishing electric fires.
Q 70. Why do you think electroplated pieces of jewellery are in demand?
Ans. Electroplated pieces of jewellery are in demand because firstly, they are as shiny and attractive as real jewellery.
They are light-weighted and cost-effective. Secondly, one feels free to wear it because of the growing problem of snatching and theft.
Q 71. Do distilled water conduct electricity? What will happen if we add sugar to it and then salt to it? Explain.
Ans. No, distilled water does not conduct electricity. If we add sugar to distilled water, then also it will not conduct electricity because sugar does not dissociate into ions. But on adding salt, it will conduct electricity because the aqueous salt solution is a good conductor of electricity.
Q 72. Suppose you want to deposit silver on an iron spoon using silver nitrate as an electrolyte. Which terminal of the battery you should connect to the spoon to? What material should the other electrode be made of?
Ans. Silver ion is positively charged, so the spoon must be connected to the negative terminal to deposit silver on it.
The other electrode should be made of silver.
Q 73. Why does potato turn green on passing current? Around which terminal greenish patch is observed?
Ans. Potato turns green due to the chemical effect of current. Around positive terminal greenish patch in potato is observed.
Q 74. Yakub made a circuit as shown in the figure. He observed that the bulb did not glow but on bringing a compass needle near it shows deflection.
He was quite confused that if the current is flowing through the circuit then why the bulb is not glowing.
Meanwhile, his friend Sourav arrived and suggested him to add one more cell in the circuit. The bulb then started glowing.

(a) Define a circuit.
(b) What does the deflection of a compass needle show?
(c) Why the bulb did not glow in the first case but glow in the second case?
(d) What value of Sourav is shown here?
Ans. (a) The circuit is a closed path through which an electric current flows.
(b) Deflection of compass needle shows that the current is flowing in the circuit. It is the magnetic effect of current.
(c) The current flowing through the circuit in the first case was too low to make the bulb glow but adding a cell in the second case makes the bulb glow.
(d) Sourav is intelligent, helpful, analytical and with scientific aptitude.