Compounds
Compounds are the substances consisting of two or more different types of elements in a fixed ratio of its atoms.
Difference between mixtures and compounds
| Compound | Mixture |
| Compounds are substances which can be formed by chemically combining two or more elements. | Mixtures are substances that are formed by physically mixing two or more substances. |
| Compounds fall under pure substances. | Mixtures can be categorized as impure substances. |
| The chemical composition of compounds is always fixed. | A mixture can have a variable composition of the substances forming it. |
| Compounds are always homogeneous in nature | Mixtures can either be homogeneous or heterogeneous in nature. |
| A new substance is formed after the constituents are chemically combined. So, a compound has different properties from its constituents. | No new substances are formed in mixtures and their properties are dependent on the properties of their respective constituents. |
Example of compounds includes water (H2O), Hydrogen Peroxide (H2O2), etc. You could see water’s chemical formula, it says it has 2 atoms of Hydrogen combined with 1 atom of oxygen and in hydrogen peroxide, it has 2 atoms of hydrogen and two atoms of oxygen.
Characteristics of compounds are as follows-
- They are homogenous.
- The properties of a compound are entirely different from its constituents.
- The constituents cannot be separated by physical methods.
- They have fixed properties like Melting point and Boiling point.
- The formation of compound is accompanied by energy changes.
Elements
- Elements are species of atoms which have the same number of protons in their atomic nuclei.
- Elements are represented by symbols e.g.Hydrogen (H), Boron (B), Carbon (C), Silicon (Si) etc.
Metals
- Metal is a solid material which is typically hard, shiny, malleable, fusible, and ductile, with good electrical and thermal conductivity.
- Examples: Aluminium, Copper, Iron, Tin, Gold
Nonmetals
- Non-metals are brittle and are not malleable or ductile.
- They are poor conductors of heat and electricity.
- Examples: Carbon, Boron etc.
Metalloids
- Metalloids exhibit some properties of metals as well as of non-metals.
- Examples: Boron, silicon, germanium, arsenic, antimony, and tellurium
Mixtures
A mixture in chemistry is a substance made up of two or more unrelated chemical components. A mixture is a physical combination of two or more distinct substances that can take the form of solutions, suspensions, or colloids.
- Crude oil: A mixture of organic compounds (mainly hydrocarbons)
- Seawater: A mixture of various salt and water.
Mixtures are formed by just mixing two or more pure substances (components) such that each substance retains its own chemical identity.

Types of mixtures
Types of Mixtures:
Heterogeneous and homogeneous mixtures are the two types of mixtures. While homogeneous mixtures seem consistent throughout, heterogeneous mixtures have clearly discernible components. A solution, which can be a solid, liquid, or gas, is the most typical kind of homogenous mixture.
Homogeneous mixture
A mixture which has a uniform composition throughout is called a homogeneous mixture or solution.
- Examples: sugar in water, salt in water.
Heterogeneous mixture
A mixture which contains physically distinct parts and has a non-uniform composition is called a heterogeneous mixture.
- Examples: Mixture of salt and iron filings, sand and sugar.
Physical & Chemical Changes
The nature of the substance, the particles that makeup it, and the quantity of particles all stay unaltered after a physical change. Chemical changes result in new compounds with different properties from the original ones, as well as new particles and may be altered particle numbers.
Physical and chemical changes
- A substance is said to undergo a physical change when only the physical properties such as the shape, size, colour or state of the substance change. No new substance is formed.
- Example: Melting of ice, boiling water.
- A substance is said to undergo a chemical change when a new substance with completely new properties (physical and chemical) is formed.
- Example: Burning of wood or paper, souring of milk.
Change
Physical Property of a Substance:
Properties of a substance such as rigidity, colour, fluidity, boiling point, melting point, density and hardness which we can observe are called as Physical Properties.
Physical Change
When physical properties of a substance change it is known as a Physical Change. When we convert a substance from one state to another, such as a solid into a liquid or vice-versa, it is also a physical change as only the physical nature of the substance changes without affecting its chemical nature.
For Example, Change of ice into water. The chemical properties of water remain the same.
Chemical Property of a Substance:
The chemical nature of a substance is known as its Chemical Property such as its odour or its chemical composition.
Chemical Change:
When the chemical properties or chemical composition of a substance gets altered it is called a chemical change. It is also called as a Chemical Reaction.
For Example, Burning of paper
Solutions
We all like drinking sweet lemon water in summers as it gives a cooling effect. It is made of lemon, water, ice, salt and sugar. These all components when mixed, form sweet lemon water mixture which is also called a solution. These components can be separated by using different techniques. Let us learn what exactly solutions are and their types.

A Solution is a homogenous mixture of two or more substances. To form it, we can add 2 or more components. These components can be in any ratio but are simply mixed, that is not chemically combined. Generally, two components of solutions are seen and are called ‘Solute’ and ‘Solvent’.
Solute
It is the constituent which is present in a comparitively lesser amount
and gets dissolved in the solvent.
Solvent
It is the constituent present in more amount and it has the ability to
dissolve the solute in it.
If you dissolve salt in water, then the salt is in lesser quantity and it gets
dissolved. So, here solute is salt and solvent is water.
Characteristics of solutions are as follows-
- They are homogeneous.
- Their Composition can vary.
- The size of particles is very small.
- They do not scatter light.
- They can be separated by physical methods.
Types of solutions
i. On the basis of dissolving nature of liquids
You must have noticed that when you dissolve sugar or salt in water, it just vanishes after a few seconds or a minute. The reason is that it gets mixed in water. But if we add oil in water, it does not vanish and is seen floating on its surface. It is due to this reason that some substances can mix into each other and some do not. Let us learn about it in detailMiscible, immiscible & partially miscible solutions

Miscible liquids
The liquids that completely mix into each other to form a solution are miscible liquids.
For example: alcohol when added to water gets completely mixed.
Partially miscible liquids
The liquids which can dissolve in another liquid only up to some extent to form a solution are partially miscible liquids.
For example: ethylene glycol in chloroform.
Immiscible liquids
The liquids which do not mix into each other are immiscible liquids.
For example: oil & water.
ii. On the basis of nature of solvent
We have seen that it’s not only water in which we can dissolve substances, we can make use of other substances as well. For examplecarbon tetrachloride, Benzene, alcohol, etc. and many more reagents. So, we have another classification based on the nature of solvent.
Aqueous and Non aqueous solutions
Aqueous
The solution in which the solvent is water is an aqueous solution.
Example: salt solution
Non-aqueous solutions
The solution in which the solvent is other than water is a non-aqueous
solution.
Example: alcohol, benzene etc.
iii. Classification on the basis of solubility power of solvent
You must have seen that if you take one glass of water at room temperature and you add 1 spoon of sugar to it, it dissolves. But if you keep on adding sugar to the same solution, a point will be reached when it stops dissolving sugar in it and the sugar starts getting deposited at the bottom. The reason being that each solvent has some solubility power and it can dissolve only up to that limit. Let us study about it.
Saturated, unsaturated and supersaturated solutions
Saturated Solution
The solution that dissolves as much solute as it is capable of dissolving is
a saturated solution.
Unsaturated solution
The solution in which more quantity of solute can be dissolved without increasing its temperature is an unsaturated solution.
Supersaturated
The solution in which the solvent dissolves an amount of solute greater than its solubility. It is formed at high temperature and then slowly cooling it to lower its solubility
iv. Classification on the basis of size of solute particles
You must have made a solution of sand in water, sugar in water and milk. They don’t look the same. Let us predict the nature of these solutions
Difference between a true solution, colloid and a suspension
| True Solution | Colloid | Suspension |
| 1) A true solution is a homogenous mixture of solute and solvent | 1) A colloid appears to be homogeneous but actually it is a heterogeneous mixture of solute and solvent | (1) A suspension is a heterogeneous mixture of a solid dispersed in a liquid or a gas. |
| (2) In a true solution the size of particle is about 10-10m | (2) In a colloid the size of particles is in between 10-7 and 10-9 m | (2) In a suspension the size of particles is greater than 10-7 m |
| (3) In a true solution the solute particles cannot be seen even with a powerful microscope | 3) In a colloid the dispersed particles can be seen with a powerful microscope | (3) In a suspension the dispersed particles can be seen with the naked eye |
| (4) The entire solution passes through filter paper | (4) The particles can pass through ordinary filter paper | (4) The particles cannot pass through filter paper |
| (5) The solute particles do not show tyndall effect | (5) The particles show tyndall effect | (5) The particles may or may not show tyndall effect |
| 6) The particles do not settle due to gravity | (6) The particles do not settle due to gravity | (6) The particles may settle due to gravity |
Colloids
Types of mixtures based on particle size
Classified into:
- Solution
- Suspension
- Colloidal Solution
A colloidal solution is a mixture in which the substances are regularly suspended in a fluid
- Classified into: Foam, Emulsion,Sol
Tyndall Effect
Tyndall effect is the scattering of light by particles in a colloid or else particles in a very fine
suspension.
- e.g.It can be observed when sunlight passes through the canopy of a dense forest.
Dispersed phase
The solute-like component of the dispersed particles in a colloid form the dispersed phase.
Dispersion medium
The component in which the dispersed phase is suspended is known as the dispersing medium
Aerosol
A colloidal solution with dispersed phase solid/liquid and dispersing medium gas is
called Aerosol. e.g. clouds
Foam
A colloidal solution with dispersed phase gas and dispersing medium solid/liquid is
called Foam. e.g. Shaving cream.
Sols
A colloidal solution with dispersed phase solid and dispersing medium liquid is
called Sol. e.g. Milk of magnesia, mud.
Gels and emulsions
- A colloidal solution with dispersed phase liquid and dispersing medium solid is called Gel.
- A colloidal solution with dispersed phase liquid and dispersing medium liquid is called Emulsion
Solutions and their properties
Anything dissolved in a solution is referred to as a solute. In a fluid solution, the amount of solvent always outweighs the amount of solute. Two of the most prevalent solutes in our daily lives are salt and water. Salt is the solute because it dissolves in water.
Examples of solute include Sugar, dissolved carbon dioxide, Oxygen, water vapour, carbon dioxide, and argon Solvent refers to the component of a solution that is most prevalent. It is the fluid in which the solute has been dissolved. Typically, a solvent is a liquid. The Latin term solve, which meaning “to loosen or untie,” is the source of the English word “solvent.”
Examples of the solvent include Water, Ethanol, Methanol, Acetone, tetrachloroethylene, Toluene, Methyl acetate, Ethyl acetate.
- A solution is a homogeneous mixture of two or more substances.
Properties:
- Its particles are too tiny and have a diameter less than 1 nm.
- The particles are not visible to naked eyes.
- Particles do not scatter a beam of light passing through it and hence do not show the Tyndall effect.
- The solute particles never settle down on keeping undisturbed.
- The components of a solution cannot be separated using filtration.
Alloys
Alloys are homogeneous mixtures of metals or a mixture of a metal and another element that cannot be separated into their components by physical methods.
Examples:
- Steel, a combination of iron (metal) and carbon (non-metal).
- Bronze, a combination of copper (metal) and tin (metal).
- Brass, a mixture of copper (metal) and zinc (metal).
Concentration of Solutions
The amount of solute that has dissolved in a specific amount of solvent or solution is measured as solution concentration. A concentrated solution is one that has a significant amount of dissolved solute in it. A diluted solution is one that has a small amount of dissolved solute in it.

Solubility
Unsaturated solutions, on the other hand, are those that contain less solute than the maximum that can be dissolved. A saturated solution is one that contains the maximum quantity of solute that can be dissolved. The amount of a solute that dissolves in a solvent is known as its solubility. The majority of solutes become more soluble when the solvent’s temperature rises.
- Solubility is the property showing the ability of a given substance, which is the solute, to dissolve in a solvent.
- It is measured in terms of the maximum amount of solute dissolved in a solvent at equilibrium.
- The resulting solution is called a saturated solution.
- Factors Affecting Solubility:
- Temperature – Solubility increases with temperature. The situation is different for gases. With the increase in temperature, they became less soluble in each other and in water, but more soluble in organic solvents.
- Pressure – For the majority of solid and liquid solutes, pressure does not affect solubility. The solubility of gas is directly proportional to the pressure of this gas.
Types of solutions based on the concentration of the solution
There are 2 main types of solutions based on the definition. Dilute Solution is a solution that contains a little amount of solute. Concentrated Solution is a solution that contains a lot of solute.
- Three types of solutions exist based on the concentration of the solution:
- Dilute Concentrated saturated solution.
Ways of representing the concentration of a solution
The concentration of a solution can be represented in many ways
(i) Mass by the mass percentage of a solution = (Mass of solute / Mass of solution) × 100
(ii) Mass by volume percentage of a solution = (Mass of solute/ volume of solution)×100
For example, if a solution of NaCl in water is said to be 10 % by volume that means a 100 ml solution will contain 10 ml NaCl.
Suspensions
Suspension and its properties
A suspension is a heterogeneous mixture in which the solute particles do not dissolve but remain suspended throughout the bulk of the medium.
- The solute particles settle down when a suspension is left undisturbed.
- They can be separated from the mixture by filtration.
- A suspension is a heterogeneous mixture.
- The size of solute particles in a suspension is quite large. It is larger than 100 mm in diameter.
- The particles of a suspension can be seen easily.
- The particles of a suspension do not pass through a filter paper. So a suspension can be separated by filtration.
Evaporation
The process of conversion of water into water vapour is known as evaporation.
Examples:
- Clothes drying in the sun.
- Tea and other hot liquids are cooled down.
- Dry Floors
- Ice cubes melting
- It can be used to separate the volatile component (solvent) from its non-volatile solute.
Introduction to Separation
Separation of components of a mixture
- Heterogeneous mixtures can be separated into their constituents by simple physical methods.
- Methods include: handpicking, sieving, filtration.

Handpicking

Sieving
Separation of Two Immiscible Liquids
Separation of a mixture of two immiscible liquids
- The separation of a mixture of two immiscible liquids is done by using a separating funnel.
- Applications: To separate a mixture of oil and water, in the extraction of iron from its ore.
Immiscible liquids break out into layers according to their densities, which is the basic idea behind the separation of immiscible liquids using a separating funnel.
Centrifugation
- Centrifugation uses centrifugal force for the separation of two liquids in a mixture.
- Here, a denser component of the mixture migrates away from the axis, and lighter component migrates towards the axis.
Applications
- Used for blood and urine tests in diagnostic facilities.
- Used to separate butter from cream in dairies and at home.
- Utilised in washing machines to extract water from drenched clothing.
Sublimation
Sublimation is the transition of a substance from solid phase to gaseous phase without changing into liquid phase.
- Example: Naphthalene balls undergo sublimation.

Solid undergoing sublimation
Chromatography
- Chromatography is used to separate the different components in a liquid mixture.
- It is based on the different properties of compounds in two phases: stationary and mobile phase.
Applications
- The technique of chromatography is extensively employed in the pharmaceutical industry in order to analyze and identify the presence of any trace amounts of chemicals and elements in a given sample.
- In the food industry, the technique of chromatography plays a vital role in the determination of the shelf life of food substances by helping in the analysis of the point at which food spoils.
- In the field of molecular biology, the study of proteomics and metabolomics often involve the use of various hyphenated chromatographic techniques (the most notable of which being EC-LC-MS).
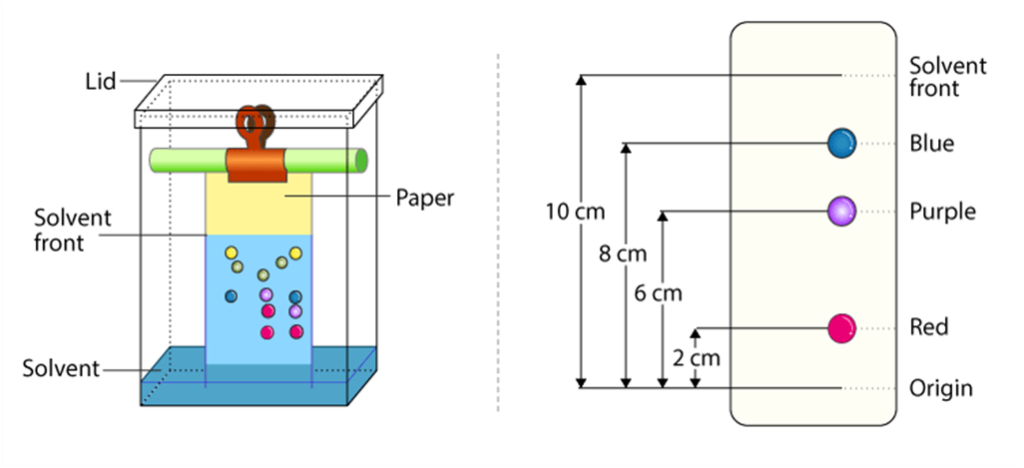
Paper Chromatography
Distillation
Distillation is a method for separating the component substances from a liquid mixture by selective evaporation and condensation.
- Used in: Production of gasoline, distilled water, xylene, alcohol, paraffin, kerosene etc.

Distillation
Fractional Distillation
Fractional Distillation is the separation of a mixture into its component parts or fractions by their melting points.
- This is the process of separation of chemical compounds by their boiling point.
- The mixture is heated to a temperature at which one or more fractions will vaporize.

Fractional Distillation
Separation of Air into Its Components
Process of obtaining different gases from the air
Air is a homogeneous mixture and can be separated into its components by fractional distillation.
Mixtures are substances made up of two or more different kinds of material. Homogeneous and heterogeneous mixtures are the two types of mixtures. There is no particle level homogeneity and the components in a heterogeneous mixture are not dispersed uniformly. As a result, we may simply divide a heterogeneous mixture into its various components.
A few popular separation methods for the heterogeneous mixture include sieving, filtration, hand-picking, etc. We must employ specialised separation procedures when dealing with homogeneous mixtures, as well as occasionally heterogeneous mixtures. Special separation techniques include evaporation, centrifugation, chromatography, sublimation, separating funnels, etc.

Fractional distillation
Crystallization
- Crystallisation is a separation technique in which solids are separated from a solution.
- In this technique, the solvent molecules start evaporating, leaving behind the solutes when the solution is heated in an open container.
Crystallisation is better than evaporation because during Evaporation. Some solids decompose or some, like sugar, may get charred on heating to dryness. Some impurities may remain dissolved in the solution even after filtration which on evaporation contaminates the solid.

Separation of substances by crystallization technique
Water Purification
- Applications of crystallisation
- Purification of seawater, separation of alum crystals from impure samples etc.
Water Purification at water works

In filtration tank water passes through different layers of sand and gravel as shown in the above figure this is for adsorption of impurities
The clear water reaches a chlorinated tank where water is mixed with bleaching powder/chlorine to kill bacteria and then supplied to houses.
Physical Change:
When physical properties of a substance change it is known as a Physical Change. When we convert a substance from one state to another, such as a solid into a liquid or vice-versa, it is also a physical change as only the physical nature of the substance changes without affecting its chemical nature.
For Example, Change of ice into water. The chemical properties of water remain the same.
Chemical Property of a Substance:
The chemical nature of a substance is known as its Chemical Property such as its odour or its chemical composition.
Chemical Change:
When the chemical properties or chemical composition of a substance gets altered it is called a chemical change. It is also called as a Chemical Reaction.
For Example, Burning of paper
