Introduction to Natural Resources
Earth is the only one on which life exists. The resources of the earth are land, water, and air. Other resources include fossil fuels, sunlight, wind, minerals, etc. Biotic factors are referred to as living things in the ecosystem. Air, water, and soil are from the non-living or abiotic components of the biosphere.
Natural Resources-Gift from nature
What are Natural Resources
Natural resources are something that comes from nature and people cannot make natural resources, but they can collect it. Some examples of natural resources are water, wood, iron, and coal. Some resources like hydroelectric energy are not natural because they are made by people.
Natural resources are classified into 2 categories:
- Renewable natural resources
- Non-Renewable natural resources
Renewable resources are those which can be generated again after it is used. For example water, wood and sunlight are some examples of renewable resources.
A non-renewable resource is that which exhausts after frequent usage and sometimes it takes a long time to get generated like natural gas. One of the examples of a non-renewable natural resource is coal. Just like coal, there are many natural resources that are limited which means it cannot be recycled again. Most of the non-renewable resources cannot be recycled and thus it is important in the conservation of natural resources. There are some natural resources that have very high demand but with less availability.
Some non-renewable natural resources are:
- Fossil fuels: Natural resources like coal, natural gas, and petroleum can be over someday i.e. they are exhaustible. It takes millions of years for a dead organism to get converted into fuels. They are consumed at a much faster rate than the rate by which it is formed. The excess burning of fossil fuels leads to air pollution as it gives out carbon dioxide which is a greenhouse gas
Conservation of Natural Resources
It is very important that these natural resources are conserved as they are getting exhausted at an alarming rate. Apart from that, it is having an adverse effect on the environment which is indirectly causing harm to live beings. By following the below tips, we can conserve natural resources:
- Minimize the use of vehicles
- Use water sparingly and do not waste water
Air and Air Pollution
An atmosphere is a layer of gases that surrounds a planet. Atmospheric air has 78% nitrogen, 21% oxygen and, 1% of other gases by volume.
Role of atmosphere
The atmosphere keeps the average temperature of earth steady. It slows down the escape of heat into outer space during the night and prevents a sudden increase in temperature during the day.
Air pollution
- Air pollution is caused by the introduction of pollutants, organic molecules, or other unsafe materials into Earth’s atmosphere
- Causes: Man-made sources include combustion of fuel, smoke from industries, Burning crackers, etc. Natural sources include forest fires, volcanoes, etc.
- Effects: Respiratory diseases, Global warming, Acid Rain, etc.
- What is Pollution?
- The change in the environment is caused by natural or artificial input of harmful contaminants into the environment and may cause instability, disruption, or harmful effects to the ecosystem.
- Thus, Pollution is essentially the introduction of toxins into the natural setting that causes negative changes. Pollution can take the form of biochemical substances or energy, such as noise, heat, or light. Contaminants, the constituents of pollution, can be one or the other, foreign substances/energies or naturally found pollutants.
What is Air Pollution?
Air pollution is defined as the introduction of pollutants, organic molecules, or other unsafe materials into the Earth’s atmosphere. This can be in the form of excessive gases like carbon dioxide and other vapors that cannot be effectively removed through natural cycles, such as the carbon cycle or the nitrogen cycle.

Types of Air Pollution
1. Man-made (Artificial) sources
These are mostly linked to the combustion of several kinds of fuel.
Immobile sources entail clouds of smoke from power plants, industrial facilities (manufacturing works), and waste furnaces, as well as incinerators and other sorts of fuel-burning heating devices. In poor and developing countries, archaic biomass burning is the chief cause of air pollution; traditional biomass embraces wood, crop leftovers, and excrement.
Movable sources comprise automobiles, aquatic vessels, and planes.
Controlled burning is a procedure sometimes used in forest management, agriculture, and prairie re-establishment. Fire is an accepted facet of both forest and grassland ecosystems and an organized fire can be an instrument for foresters. Precise burning kindles the sprouting of some desirable trees, thus renewing the forest.
Fumes from hair spray, paint, aerosol sprays, varnish, and other solvents
Waste deposits in landfills create methane. Methane is extremely combustible and may form an explosive and volatile concoction with air. Methane is furthermore an asphyxiant and may displace oxygen in a sealed-off space. Suffocation may result if the oxygen concentration goes below 19.5% by displacement.
Military resources, such as nuclear weapons, toxic gasses, germ warfare and rocketry
2. Natural sources
- Dirt from natural sources, typically big areas of land with little or no plant life.
- Methane is discharged by the breakdown of food (digestion) by animals, for example, cattle
- Smoke and CO from jungle fires.
- Volcanic activity, which emits sulfur, chlorine, and ash particulates
- Causes of Major Effects of Air Pollution
- Air pollution has a very negative effect on humans and the ecosystem. The constituents can be dense particles, fluid, or gasses. A contaminant can be of natural or artificial. Contaminants are categorized as primary or secondary.

Primary contaminants are typically created by the emission of carbon dioxide from vehicles and factories. Secondary pollutants are contaminants that are not emitted directly into the atmosphere. They are formed in the atmosphere when prime pollutants react or intermingle. Ground-level ozone is an important example of a secondary pollutant.
Some contaminants may be both primary and secondary: they are both emitted directly and formed from other primary pollutants.
Effects of Air pollution
- Respirational and cardio complications: The adverse impacts of Air pollution are distressing. They are the root of numerous respirational and cardiac conditions accompanied by Cancer, midst other threats to our body. More than a few million are known to have expired due to direct or unforeseen effects of Air contamination. Kids in areas open to air contaminants are said to suffer frequently from pneumonia and asthma.
- Global warming: One more direct consequence is the speedy changes that the world is observing due to Global warming. With the increase in temperatures worldwide, an increase in sea levels, and melting of ice from cold areas and icebergs, displacement and loss of habitat have already beckoned an imminent disaster if actions for protection and regulation aren’t undertaken soon.
- Acid Rain: Dangerous gasses like NO2 and SO2 are released into the atmosphere during the incineration of fuels. When it rains, the droplets combine with these pollutants, become acidic and then fall on the ground in the form of acid rain. Acid rain is a source of great harm to human beings, wildlife, and crops.
- Eutrophication: It is a process where a high amount of nitrogen present in some contaminants gets morphs on the sea’s surface and develops itself into algae and harmfully affects fish, plant life, and animal species. The green-colored algae that are present in lakes and ponds are due to the presence of this substance only.
- Diminution of Ozone layer: Ozone is present in the Earth’s atmosphere (Stratosphere) and is responsible for shielding humans from injurious ultraviolet (UV) rays. Earth’s ozone layer is diminishing because of the presence of chlorofluorocarbons and hydrochlorofluorocarbons in the atmosphere.
Prevention of Air Pollution
Some important measures that can be adopted by individuals to contribute towards the prevention of air pollution have been listed below.
- Usage of public transport and carpooling
- Switching off the lights when they’re not in use
- Reusing and recycling products
- Avoiding the burning of garbage and smoking
- Avoiding the use of firecrackers
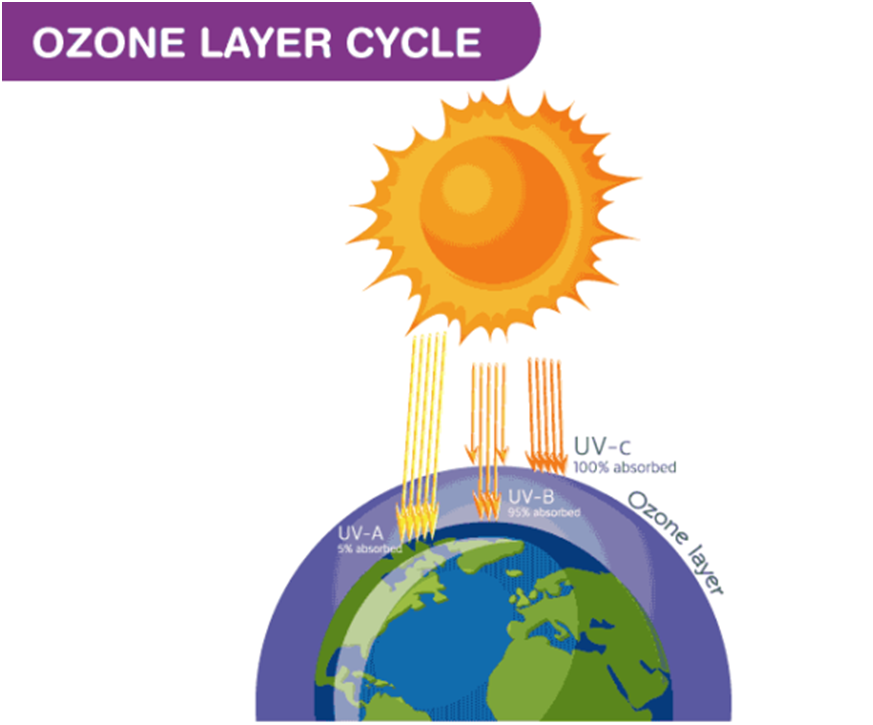
Ozone layer
The ozone layer is a thin part of the Earth’s atmosphere, which functions as a shield over the Earth’s stratosphere and absorbs the greatest amount of the Sun’s ultraviolet (UV) radiation. The ozone layer comprises high concentrations of ozone (O3) in relation to other parts of the atmosphere.
What is the Ozone Layer?
Almost always the ozone has only been correlated with the hole in the ozone layer and the damage it has caused to the environment. The richness of the ozone layer makes the hole so significant and the science behind the hole is far less popular. Schönbein in the year 1840 confirmed its existence and Jacques-Louis Soret rooted the chemical formula of ozone as O3 and proved that ozone is an allotropic form of Oxygen.
Preparation of Ozone
This allotropic form of oxygen is formed by passing dry oxygen through a salient electric with the oxygen molecule to give 5-10% of the allotropic form of oxygen. The product obtained is called ozonized oxygen.
\(\begin{array}{l}O_{2}\overset{Energy}{\rightarrow}O + O\end{array} \)
O2+O → O3
3O2 2O3 – energy (endothermic reaction)
Ozone is unstable and decomposes to molecular oxygen. A dynamic equilibrium is maintained between the formation and decomposition of ozone. It has been found that this protective ozone layer is getting depleted because of the presence of CFC (chlorofluorocarbon) compounds.
When CFC is released into the atmosphere, they mix with atmospheric gases and reach the stratosphere. In the presence of ultraviolet rays, they are broken down to form chlorine radicals. This chlorine radical reacts with ozone to form chlorine monoxide and an oxygen molecule.
CF2Cl2 (g)
Cl (g) + CF2Cl (g)
Cl (g) + O3 (g) → ClO (g) + O2 (g)
This reaction breaks down the ozone. CFC compounds are agents which release chlorine radicals in the atmosphere and cause damage to the ozone layer.
Ozone is a polar molecule and to understand this we need to have a look at the structure of Ozone. Ozone resonates between two structures which are shown below:

The middle Oxygen atom has a formal charge of +1 and the atoms at the edge have a formal charge of -1. Due to the separation of light charges and its bent geometry, it has polarity and is considered a polar molecular.
Properties of ozone
- Ozone in its pure state is blue which has a strong disturbing smell but in a limited proposition, it has a pleasant smell.
- It has the ability to absorb the UV rays which occupy the ultraviolet region which ranges between 220-290 nm of the atmospheric spectrum.
- This form of oxygen boils at 161.2K and forms violet-blue crystals when solidified. It melts at 80.6k.
- This allotrope is a strong oxidizing agent as ozone is an unstable compound under normal conditions and it decomposes quickly in the presence of heat to form nascent oxygen and molecule of oxygen.
- Importance of the ozone layer
- Ozone is harmful at ground level but high up the atmosphere ozone layer plays a vital role in the protection of all living beings. The sun propagates ultraviolet radiation which has an adverse effect on living beings. This layer absorbs the radiations and prohibits them from entering the outer surface of the earth. The ozone layer resides in the stratospheric layer of the earth’s atmosphere. The layers which occupy the lower part of the atmosphere remove the unwanted pollutants from the earth’s surface.
Ozone layer depletion

The reason behind the ozone layer depletion is mainly due to the extensive use of ozone-depleting substances (ODS). Some ozone-depleting substances are:
- Chlorofluorocarbons (CFC): The use of CFCs is one of the main reasons for the depletion of the layer. They are usually used as a coolant in refrigerators and air conditioners used in cars etc. It is also used as an industrial solvent, foam products, and as hospital sterilization equipment.
- Methyl chloroform: Finds its applications usually in industries for chemical processing etc.
- Carbon tetrachloride: Normally used as a solvent.
Ozone layer depletion
Ozone layer depletion is the reduction of the amount of ozone in the stratosphere which results in greater UV radiations reaching the earth’s surface.
CFCs
Chlorofluorocarbon (CFC) is an organic compound that contains carbon, chlorine and fluorine
Greenhouse effect
The greenhouse effect is a natural phenomenon, which occurs when the greenhouse gases present in the Earth’s atmosphere trap solar radiation. Carbon dioxide (CO2), methane (CH4), ozone (O3), chlorofluorocarbons (CFCs), nitrous oxide (N2O), and water vapor (H2O) are called greenhouse gases.
Water: A natural resource
Role of water in everyday life: Water forms two-thirds of our body, it keeps the body’s temperature normal. It is also used for agricultural purposes, Domestic Purposes, Industrial Purposes, etc. Distribution of water on earth: Only 3% of the water on the surface is fresh, the remaining 97% resides in the ocean.
Water pollution
Water pollution is the contamination of water bodies, caused by discharging pollutants directly or indirectly into the fresh and clean water bodies without adequate treatment.
The main causes of water pollution are
- Urbanization.
- Industries
- Agriculture
- Religious and Social Practices
- Withdrawal of water and drying up of water bodies
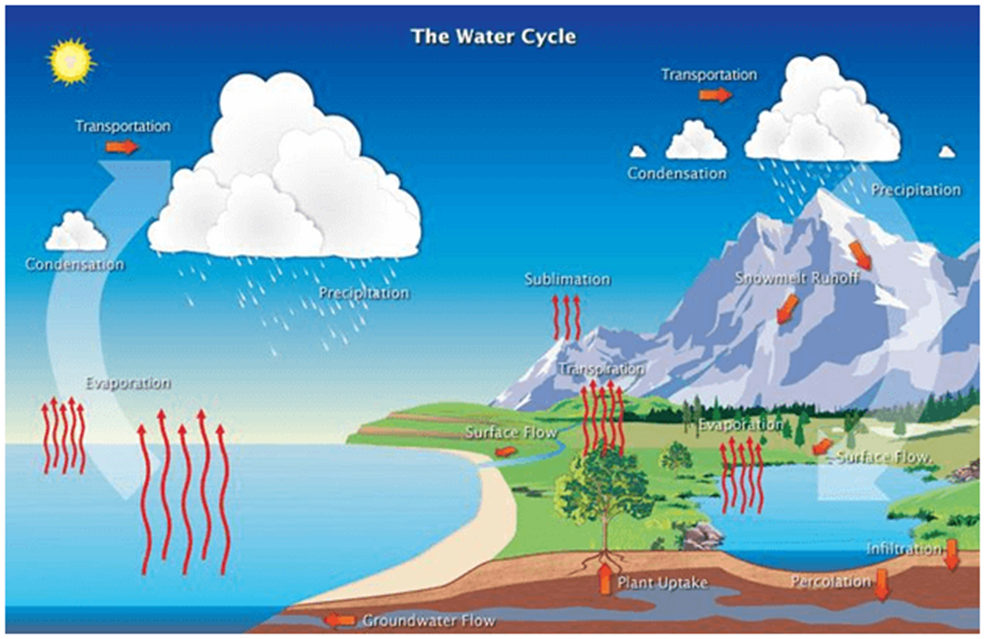
Water cycle
- The water cycle, also known as the hydrologic cycle, is the continuous movement of water from the earth’s surface to the atmosphere and then back to the ground.
Soil
Soil and its formation
Soil is the uppermost layer of the Earth’s crust, formed by the continuous weathering of mountains. Factors causing soil formation are:- Parent material, Time, Climate, and Organisms.
Soil composition
Soil is a mixture of organic matter. The basic components of soil are minerals, inorganic matter, water, and air. Various types of soil are clay, loam, silt, sand, etc.
Humus
The organic constituents including the dried leaves, twigs, and remains of plants and animals decompose to form the upper organic layer, known as humus. It plays an important role in increasing the fertility of the soil.
Soil pollution
The addition of harmful or toxic chemicals to the soil which renders it unproductive is called soil pollution. Fertilizers, insecticides, industrial wastes, accidental oil spills, acid rain, etc. are pollutants and are the main causes of soil pollution.
Soil erosion
Soil erosion is one form of soil degradation. Flowing water, rainwater, and the wind are the prime agents which cause soil erosion. This causes loss of topsoil and also reduces crop production potential.
Bio-geo-chemical cycle
The natural cycle or pathways in which the essential matter is circulated through the biotic and abiotic parts of an ecosystem.
- Biogeochemical = Biological Chemical + Geological Process
To know more about Biogeochemical Cycle, visit here.
Carbon
Carbon is a chemical element with the symbol C. It is a nonmetallic chemical element, found in various forms:
- In Elemental forms- Diamond, graphite
- In Combined form-carbon dioxide, carbonates
- Carbon-containing molecules are proteins, carbohydrates, fats, nucleic acids, vitamins
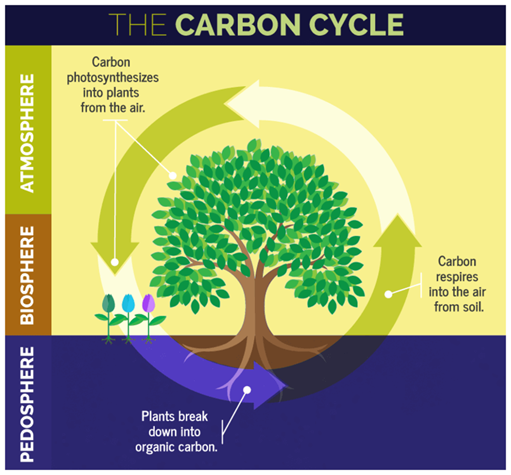
Carbon cycle
The circulation and transformation of carbon between living things and the environment is called the Carbon Cycle.
Nitrogen Cycle
The nitrogen cycle is the recycling and reusing of nitrogen in different forms to meet the demands for various environmental activities.

Oxygen Cycle
It is a biological process that helps in maintaining the oxygen level.
Photosynthesis is a biological process used by plants to prepare their food with the help of sunlight and energy.
